Gujja A.A1. MA’. Aza, A.A2 Babagana U3, Tarimbuka L.Y.4
1Department of Forestry Technology, Yobe state college of Agriculture, Science and Technology Gujba P.M.B 1104 Damaturu, Nigeria. Email address akiagujja2465@gmail.com
2Department of laboratory science technology, Yobe state college of Agriculture, Science and Technology Gujba P.M.B 1104 Damaturu, Nigeria. Email, muazualkaliadamu@gmail.com
3Department of laboratory science technology, Yobe state college of Agriculture, Science and Technology Gujba P.M.B 1104 Damaturu, Nigeria. 4 Department 0f Basic Science, Adamawa State College of Agriculture PMB Ganye, Adamawa State.
| ARTICLE INFORMATION | ABSTRACT |
| *Corresponding author: GUJJA A.A. E-mail: akiagujja2465@gmail.com Keywords: Date palm Microorganism Microbial isolates Variability | This research assessed the microbial isolates in the soil of the date palm plantation of Modibbo Adama University, Yola, Adamawa State. Parameters evaluated included; microbial isolates based on the growth performances of the date palm. The plantation was divided according to growth variabilities. Fifteen (15) auger points were taken, five (5) in each performance site based on the corresponding variability as observed. Soil samples were collected within the same points for analysis of microbial isolates, Nutrient agar medium at 105 dilutions was inoculated in a petri dishes and incubated at 300C±10°C for 2-5 days for bacteria colonies, while for fungi and actinomycetes, sabaurond dextrose agar was used at 25°C for 5-7 days and afterward microorganisms per colony forming units (Cfu) were counted. Results from the study area showed that the area is highly rich in microorganisms. A total of 5096 colonies were scattered within 10 families in the study area. Some microorganisms identified were Actinomyces crime, Aspergillus niger, Staphylococcus aurus, Streptococcus species, Pseudomonas auroginosa, Escherichia coli, Bacillus subtilis, and Lactobacillus species among others. The finding of this study revealed that there was no significant difference among the microorganism across the study site leading to the conclusion that soil microorganisms were present in the study area. However, the microorganisms were very active in the date palm plantation soil. This is an indication that a strong relationship exists between, microorganisms and the date palm trees. |
INTRODUCTION
Soil contains many micro flora and fauna as long as there is a carbon source for energy. A large number of bacteria in the soil exist, but because of their small size, they have a smaller biomass. Actinomyces are 10 times smaller in number but are larger in size so they are similar in biomass to bacteria in soil (Bhattarai, et al. 2015; Roohallah et al. 2022).
and often critical roles in these ecosystem services. The vast metabolic diversity of soil microbes means their activities drive or contribute to the cycling of all major elements (e.g. C, N, P), and this cycling affects the structure and the functions of soil ecosystems as well as the ability of soils to provide services to people provisioning and regulating ecosystem services (Suzanne ,2017).
Plant and animal detritus and root exudates represent essential sources of energy and nutrients for soil microbial and faunal communities. Bacteria and fungi represent 95% of the biomass present in most soils, where they interact with a combination of micro-fauna (nematodes, protozoa), Meso-fauna (acari, Collembola, mites) and macro-fauna (earthworms, termites, molluscs) in complex soil food-web systems that determine the turnover of organic matter and associated nutrients in the soil environment ( Moghimian and Kooch, 2013; Duran et al. 2019).
Decomposition of organic carbon in soil is driven primarily by the activities of bacteria and fungi, while only 10–15% of soil carbon flux can be directly attributed to the actions of fauna (Shang et al. 2017). The vast majority of soil microorganisms are heterotrophs that rely on organic matter for energy and nutrients.
These can be divided into microorganisms that respond primarily to the addition of fresh carbon substrates (zymogenous or r selected biomass) and those that derive their energy mainly from the decomposition of older, more recalcitrant forms of organic carbon (autochthonous or K selected biomass) (Shang et al. 2017).
The date palm (Phoenix dactylifera L.) tree belongs to the family Arecaceae and is considered a symbol of life in the desert, as it tolerates high temperatures, water stress, and salinity more than many other fruit crops (Effi, et al. 2011). Date palms can be planted in a wide range of soils with varying amounts of organic and mineral nutrients.
Many parts of the world where date palm is grown still follow the traditional mixed planting of dates of various ages at irregular spacing. Moreover, inadequate fertilizer application and lack of proper tree and bunch management, such as pruning and fruit thinning, lead to the production of low fruit quality and thus lower market values (Elamin et al. 2017).
In the five years of the establishment of the date palm plantation of Modibbo Adama University of Yola, studies have not been done on biological constituents of the soil. The growth of the individual date palm plants have not been uniform, while some are performing very well, others have indication of stunted growth.
Since the growth of every plant depends largely on the nutrient status which is in turn affected by the activities of soil microorganism, information on the soil biological components in the date palm plantation becomes a pre requisite to understanding the differences in the performances of individual plants. The aim of this study is to assess the microbial isolates in the study area. The specific objectives are to identify and evaluate the microbial isolates in the study area. At the end of the study the biological component of the date palm plantation has been ascertained.
The information on microorganism will thus, baseline information for future management of the date palm plantation soils and by extension any other date palm that may be grown under similar conditions. The results of the research will be an invaluable tool to the date palm plantation managers in the Department of Forestry and Wildlife Management, Modibbo Adama University of Yola and indeed many other organizations and individuals that are involved in date palm research and production.
MATERIALS AND METHODS
The Study Area
Adamawa State is located in the North Eastern part of Nigeria. It lies between latitude 7o and 11o N of the equator and longitude 11o and 14o E (Figure 1) (Adebayo et al., 2020). The date palm plantation of the Department of Forestry and Wildlife Management Modibbo Adama University, Yola, Adamawa State is located between latitude 8°N and 11°N Longitude 11.5°E and 13.5°E (Figure 2).
Adamawa state falls under the Sudan, southern and Guinea savannah types of vegetation and its experiences distinct dry and wet seasons with temperature and humidity varying with seasons.
The wet or rainy season falls between April and November, which is characterized by a single maximum in August and September. During this season, the moisture-laden southwest trade wind from the Atlantic Ocean blows over the area. Seventy percent of the total rainfall in the area happen to fall within four month of May- September (Adebayo et al. 2020).
The area has an average of 62 rainy days, while average amount of rainfall recorded in the area is 972 mm the dry season which is the harmattan period between December March. The period is characterized by dry, dusty and hazy northern trade wind that blows over the area from Sahara desert.
Temperature within the area varies with season. Although the temperatures are relatively high almost all the year round, temperature of the area ranges from 27°C-40° C. December and January is the coldest months with the average temperature of 34° C (Adebayo, et al. 2020). The natural vegetation of the area is Sudan savannah type which is characterized by thick vegetation around hills and mountain ranges.
The vegetation has a wide variety of savannah trees species among which area are; Acacia spp, Adansonia spp, Anogeisus spp. (Akosim et al. 2020).
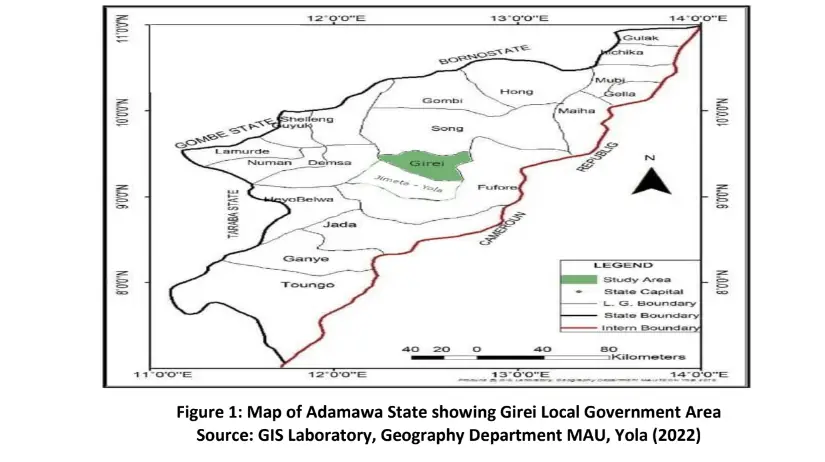
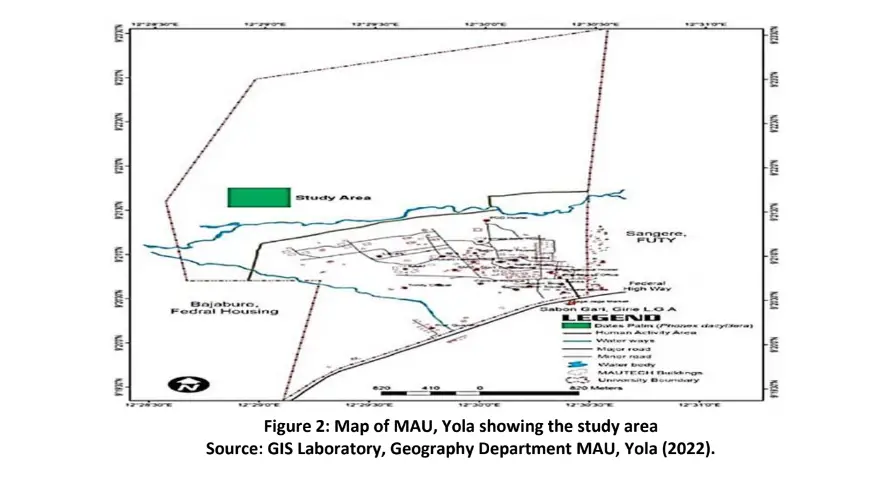
The soils of Adamawa State are classified as ferruginous tropical soils. These types of soils are defined often generally as having a marked differentiation of horizons and an abundance of free iron oxides usually deposited as red or yellow mottles or concretions. The soils of Adamawa State as derived from this system include Luvisols, Legosols, Cambisols, Vertisols and Lithosols (Adebayo et al. 2020).
Soil sampling process for microbial isolates
Soil sample was taken in each auger point in the date palm plantation. A composite sample, after mixing the sample thoroughly, sterile polythene bags was used to convey samples to the laboratory within 24 hours of collection for analysis of soil bacteria, fungi, and Actinomycetes at the department of microbiology of Modibbo Adama University, Yola.
Culture
Bacteria population was estimated by the method of Vieira, (2005) using the nutrient agar medium at 105 dilutions. The inoculated petri-dishes was incubated at 300c±10°c for 2-5 days for bacteria colonies. The laboratory analysis involved adding 1g of soil into 9ml of sterile water in a test tube, followed by vigorous shaking, and then serial dilution was done in four test tubes before transfer into the petri dish.
However, molten agar/media was poured into the petri dish. For isolation and characterizing of fungi and Actinomycetes dilution plate method was used, sabaurond dextrose agar for fungi and Actinomycetes selected media for Actinomycetes was used as basal medium to isolate species.
The inoculated petri- dish was incubated at 25°c for 5-7 days for growing the fungi and Actinomycetes colonies. Representative isolates of fungi was identified under the microscope with the help of standard manuals (Naher et al. 2013).
Representative isolates of bacteria were also identified under the microscope. Fungi identification was done under the appearance and pigmentation of spores on agar and Actinomycetes was identified under the appearance on agar plates.
RESULTS
Soil Microorganisms (cfu-1) in the Study Areas
The soil micro – organisms ‘colony count result shows the presence of Actinomycetaceae, (Actinomyces cream, Actinomyces yellow, Actinomyces blue), Staphylococcaceae; (Staphylococcus aureus), Streptococcaceae, (Streptococcus spp.), Pseudomonadaceae, (Pseudomonas auroginosa), Enterobacteriaceae, (Escherichia coli), Bacillaceae, (Bacillus subtilis), Trichocomacaceae, (Aspergillus niger, Aspergillus fumigates), Lactobacillaceae, (Lactobacillus spp.), Aeromonadaceae, (Aeromonas spp.), Streptomycetaceae, (Streptomyces spp.), Enterobacteriaceae, (Klebsiilla spp., Proteus spp., Citrobacter spp.), and Bacillaceae, (Bacillus copus). The soil micro – organism’s colony where categorized into three (3) basic forms Bacteria, Fungi and Actinomycetes.
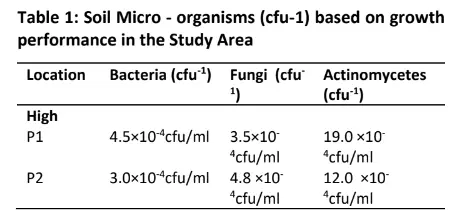
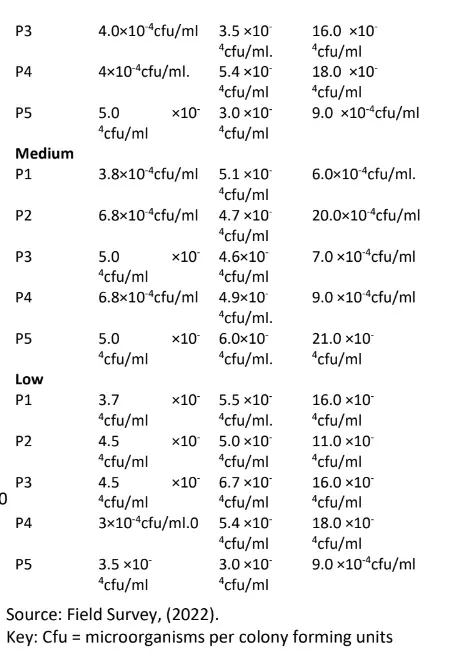
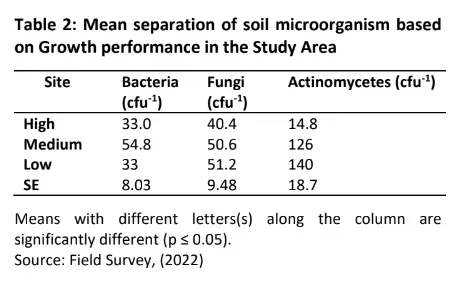
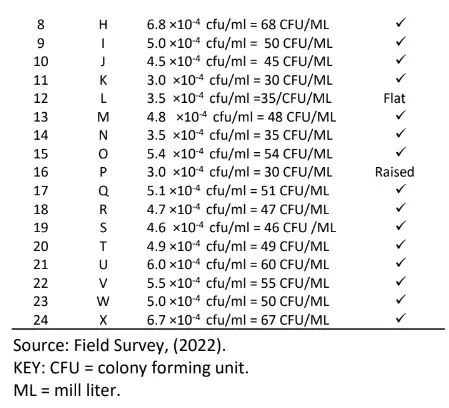
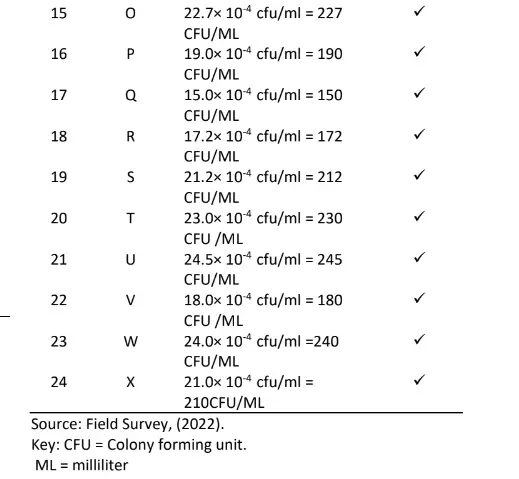
The result of the soil micro – organism’s colony count (cfu 1) based on high growth performance in the study area is presented in Table 1. The results of the high growth performance site indicate that Bacteria count ranged from 3.0×10-4cfu/ml to 5.0 ×10-4cfu/ml, Fungi count ranged from 3.0 ×10-4cfu/ml to 5.4 ×10-4cfu/ml and Actinomycetes count also ranged from 9.0 ×10-4cfu/ml to 19.0 ×10-4cfu/ml respectively.
Results of the medium growth performance site indicate that Bacteria count ranged from 3.8×10-4cfu/ml to 6.8 ×10-4cfu/ml, Fungi count ranged from 4.6 ×10-4cfu/ml to 6.0 ×10-4cfu/ml and Actinomycetes count also ranged from 6.0 ×10-4cfu/ml to 21.0 ×10-4cfu/ml respectively.
From the low-performance site, results indicate that Bacteria count ranged from 3.0×10-4cfu/ml to 4.5 ×10– 4cfu/ml, Fungi count ranged from 3.0 ×10-4cfu/ml to 6.7 respectively.
1.1 Checklist of Micro-Organisms in the Study Area
Table 3 shows the checklist of eleven (11) families containing seventeen (17) species of microorganisms identified in the study area. The families encountered were Actinomycetaceae (Actinomyces crime, Actinomyces yellow, Actinomyces blue), Aeromonadaceae (Aeromonas spp.), Trichocomacaceae (Aspergillus niger, Aspergillus fumigatus),
Staphylococcaceae (Staphylococcus aurus), Streptococcaceae (Streptococcus spp.), Pseudomonadaceae (Pseudomonas auroginosa), Enterobacteriaceae (Escherichia coli, Klebsiilla spp.,` Citrobacter spp., and Proteus spp.), Bacillaceae (Bacillus subtilis, Bacillus copus), Lactobacillaceae (Lactobacillus spp.), Aeromonadaceae (Aeromonas spp.) and Streptomycetaceae (Streptomyces spp.) were present in the plantation soils..
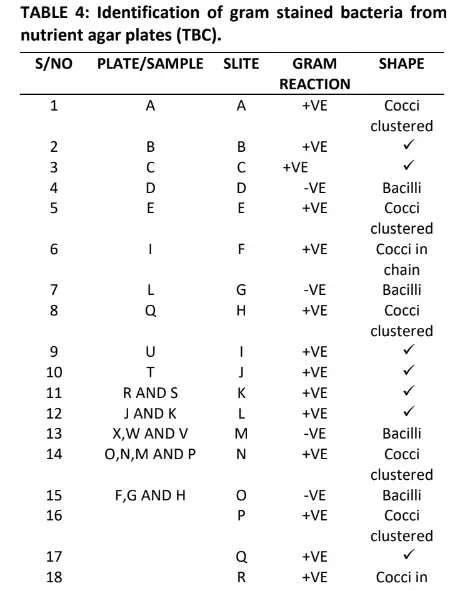
The result in Table 4 shows the identified gram stained bacteria from nutrient agar plates which five 5 indicate gram negative and nineteen (19) show gram positive in term of shapes the result indicates fifteen (15) Cocci cluster, five (5) bacilli, and four (4) Cocci in chain respectively.
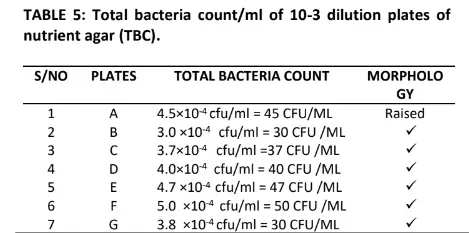
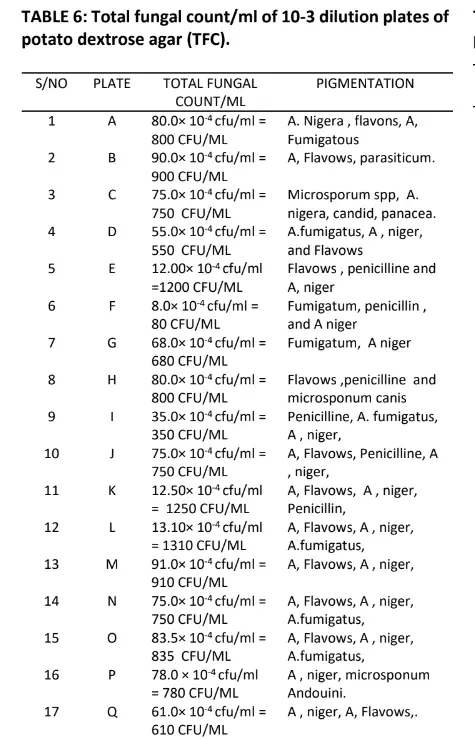
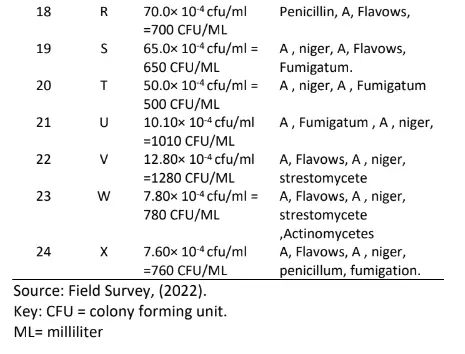
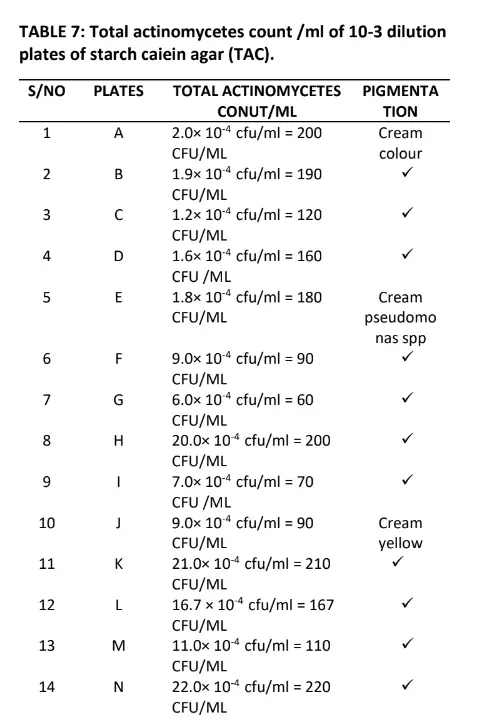
Table 5 also shows the morphology of the bacteria in which four indicated flat and twenty raised respectively. The result in table 6 indicates the total fungal diluted in plates of potato dextrose agar, the pigmentation shows Aspergillus niger, Aspergillus fumigatus, Penicillin, Flavows, Microsponum canis, Microsponum andoumis, Strestomycete and Actinomycetes respectively. The result in Table 7 shows the total Actinomycetes counts in diluted plates of starch casein agar, the pigmentation shows four cream colour, five cream pseudomonas species and fifteen cream yellow respectively.

DISCUSSION
Soil Microorganisms (cfu-1) in the Study Areas
Findings of the soil microorganisms showed a wide diversity in the date palm plantation which were categorized based on high, medium and low performance sites.
These microorganisms (bacteria, fungi and actinomycetes) influence plant diversity and productivity according to van der Heijden et al. (2008). This is because they play important roles in the nutrient cycles and energy flows, providing essential services to the forest ecosystem. Soil fungi, for example have the function of catalyzing the turnover of complex organic resources, which can drive the degradation of organic matter. Bacteria generally utilize the easily available substrates decomposed by fungi.
Conversely, they are affected by the plant communities as they depend on the products of plant photosynthesis: litter and rhizo-deposits (Wardle, 2006; Qiao et al., 2014; Prescott and Grayston, 2013). The microorganisms ‘influence on plant diversity and productivity is in consonance with the findings of Lladó, et al. (2017) who stated that bacteria commonly harbor genes encoding plant cell wall-degrading enzymes and contribute significantly to the decomposition of organic matter.
In addition, bacteria are the major natural agents responsible for N fixation in forest ecosystems and for other ecosystem processes, such as mineral weathering leading to the release of inorganic nutrients. The roles of bacteria and fungi, however, should not be viewed as separate.
The high abundance of fungal biomass in forest soils has multiple consequences for bacteria, including the creation of specific niches in the soil patches colonized by mycorrhizal fungi (i.e., the mycorrhizosphere) and soil mycelial mats, provision of nutrients via organic matter decomposition, and an increase in soil connectivity by fungal mycelia that allow certain bacteria to move across the environment.
Also, the fining shows that the numbers of bacterial communities decreases with decrease in depth. This agrees with the report of Lauber et al. 2009; Rousk et al. (2010) stating that bacterial abundance and diversity have been reported to decrease with decreasing soil pH. Similarly, the composition of fungal communities has been previously shown to differ substantially between litter and organic horizons, while deeper soil horizons showed greater similarity (O’Brien et al. 2009; Lindahl et al. 2007).
In several forest types, this is due to the higher abundance of saprotrophic fungi in litter and the dominance of ectomycorrhizal species in deeper soil (Lindahl et al. 2007; Edwards et al. 2010).. Duran et al. (2019) stated that date palm can influence the composition and functioning of the soil bacterial community by altering the microclimate (via shading and through fall effects and uptake/transpiration of soil water), litter production, amount and quality of root exudates, and interactions with root symbiotic organisms such as mycorrhizal fungi.
This is because, in the forest ecosystem, trees can change the forest microclimate, and they can produce exudation from roots, litter, and wood debris; meanwhile, they (trees) interact with soil microbes and micro fauna through roots, and thus, can influence ecosystem properties.
Date Palm can selectively attract and maintain rhizosphere microbes by root exudates, and at the same time, the microbial communities may strongly affect the growth of date palm by releasing mineral elements. Thus, the interaction between aboveground vegetation and soil microbial communities can influence the process of the forest ecosystem.
Findings also show the presence of highly important bacterial and fungal groups (though in few numbers). In date palm plantation soils, microbial communities are affected by the changes in aboveground vegetation communities and soil environmental properties (such as nutrients, temperature, and moisture) influenced by human activity. These changes (as a result of agricultural activities) in the date palm plantation are believed to be responsible for the relatively low density of microorganisms in the study area. The loss of important microorganism as a result of application of pesticides in the plantation this also agreed with the findings of Mikayla et al. (2017) who stated that plantation could induce significant shifts in soil microbial community through biotic and abiotic factors, including species composition, above- and below ground litter, and soil substrate quality and quantity, which are associated closely with soil microbial community.
CONCLUSION
Based on Based on the findings of this study, it can be concluded that; microorganisms were present in the study area. However, the numbers of microorganisms were very active in the date palm plantation soil. This is an indication that strong relationship exists between microorganisms and the date palm trees. The organism’s breakdown complex organic matter, giving fertility to the soil, others like Actinomycetaceae through the ground, making the soil fertile and nutrients pass through and absorbed by the date palm trees. The date palm trees on the other hand produce food for microorganism through litters and exudates.
RECOMMENDATIONS
The following recommendations are hereby made:
The Agricultural practices which combine trees and crops should be encouraged and application of agrochemicals is deleterious to the interrelationship of microorganisms and date palm plants. It also interferes with the varieties of food chains and food webs of the ecosystem.
REFERENCES
Adebayo, A. A., Tukur A. L. and Zemba, A. A. (2020). Adamawa State in Maps. Paraclete Publishers Yola, Nigeria. Pp. 20-22.
Adebayo, A.A. (2020) Climate, sunshine, temperature, evaporation and relative humidity. In Adamawa state in map.
Adrina, L Gusmini, A Darfis S, Elsa L. and Putri ,G (2021) Performance of Some Soil Physical Properties of Arabica Coffee Plantation in Solok Regency IOP Conference. Series: Earth and Environmental Science 741 (2021) 012028 IOP Publishing doi:10.1088/1755-1315/741/1/012028
Akosim, C., Tella, I. O. and Jatau, D. F. (2020). Vegetation’s characteristics of Adamawa State. In: Adebayo, A. A.,
Tukur A. L. and Zemba, A. A. Adamawa State in Maps. Paraclete Publishers Yola, Nigeria. Pp. 20-22. Bhattarai A, Bishwoyog B and Pandey S (2015) Variation of Soil Microbial Population in Different Soil Horizons Journal of Microbiology and Experimentation Volume 2 Issue 2 – 2015
Claridge, A. W., Trappe, J. M., and Hansen, K. (2009). Do fungi have a role as soil stabilizers and remediators after forest fire?. Forest ecology and management, 257(3), 1063-1069.
Duran, P.; Barra, P.J.; Jorquera, M.A.; Viscardi, S.; Fernandez, C.; Paz, C.; Mora, M.D. and Boll, R.(2019) Occurrence of Soil Fungi in Antarctic Pristine Environments. Front. Bioengineering. Biotechnology, 7, 28. [CrossRef]
Edwards I P , Zak D R McGuire K L. C B. Blackwood and Rima U (2010) Slowed decomposition is biotically mediated in an ectomycorrhizal, tropical rain forest Springer-Verlag 2010
Effi, T, Uri, S , Yechezkel, M. and Alon, BG.(2011) Long term growth, water consumption and yield of date palm as a function of salinity. Journal of agricultural water management 99(1)128-134.
Elamin A.H , Elsa dig ,E.H .Alijubouri,H.J , Gafar ,M.D (2017) Improving fruit quality and yield of khenazi date palm (phoenix dactylifera L) growth in sandy soil by application of nitrogen ,phosphorus, potassium and organic matter .international journal of development and sustainability 6 (8)862- 875.
Gholami, S., Sheikhmohamadi, B. and Sayad, E. (2017). Spatial relationship between soil macro fauna biodiversity and trees in Zagros forests, Iran. CATENA. 159. 1-8. 10.1016/j.catena.2017.07.021.
Geographical Information System (2022) Laboratory, Geography Department Modibbo Adama University, Yola Adamawa State Nigeria
Holden, S. R., and Treseder, K. K. (2013). A meta-analysis of soil microbial biomass responses to forest disturbances. Frontiers in microbiology, 4, 163.
Juan F. G, Vicki H. W, Brian M. M. A and Kelly C. W. (2017) Chemical and pathogen-induced inflammation disrupt the murine intestinal micro biome volume 5, Article number: 47 springer link.
Kabir, M. A. (2020). Evaluation of Biodiversity Status and the Supporting Systems in Relation to Socio Economic Characteristics in Lakeshore Communities of Kainji Lake National Park, Nigeria. An Unpublished Ph.D. Thesis, Submitted to the Department of Forestry and Wildlife Modibbo Adama University, Yola. (MAUTECH).
Kekane,S.S, Chavan, R.P, Shinde, D.N, Patil, C.L and Sagar, S.S (2015) A review on physico-chemical properties of soil International Journal of Chemical Studies P-ISSN 2349–8528 E-ISSN 2321–4902 IJCS 2015; 3(4): 29-32.
Khan Towhid Osman, (2016) Plant Nutrients and Soil Fertility Management,” in Soils: Principles, Properties and Management, ed. Khan Towhid Osman (Dordrecht: Springer Netherlands, 2016), 129–59, https://doi.org/10.1007/978-94-007-5663- 2_10.
Korboulewsky, N., Perez, G., and Chauvat, M. (2016). How tree diversity affects soil fauna diversity: a review. Soil Biology and Biochemistry, 94, 94-106
Lauber, C. L., Hamady, M., Knight, R., and Fierer, N. (2009). Pyro sequencing-based assessment of soil pH as a predictor of soil bacterial community structure at the continental scale. Appl. Environ. Microbial., 75(15), 5111-5120.
Lindahl, B D. Katarina I, Johanna B, Susan E. T, Peter H, Jan S, Roger D. F(2006) Spatial separation of litter decomposition and mycorrhizal nitrogen uptake in a boreal forest https://doi.org/10.1111/j.1469- 8137.2006.01936.xVolume 173, Issue 3 Pages: 447- 660
Lladó, S., López-Mondéjar, R., and Baldrian, P. (2017). Forest soil bacteria: diversity, involvement in ecosystem processes, and response to global change. Microbiology and Molecular Biology Reviews, 81(2), e00063-16
Lucas-Borja, M.E, de Santiago, J.H, Yang Y, Shen Y, and Candel-Pérez D (2018) Nutrient, metal contents and microbiological properties of litter and soil along a tree age gradient in Mediterranean forest ecosystems. Science Total Environment 650:749– 758. Doi :https://doi.org/10.1016/j.scitotenv.2018.09.079
Mikayla A. B, Anice S, Jikang W, Lindsey M. S, Bridget S. O, Rebecca A. D, Richard A. W, Moghimian, N., and Kooch, Y., (2013). The effect some of physiographic factor and soil physic- chemical features of Hornbeam forest ecosystem on earthworm biomass. Journal of wood and Forest Science and Technology 20(2): 1-21.
Naher, A. U .Othman, R, and Panhwer, A.Q (2013) Culturable total and beneficial microbial occurrences in long-term nutrient deficit wetland rice soil. Australian journals of crop science AJCS 7(12):1848-1853.
O’Brien H E , Jeri L P, Jason A J, Jean- M, and Rytas V. (2005) fungal community analysis by large-scale sequencing of environmental samples. Applied environmental microbiology 2005 Sep; 71(9):5544- 50.doi: 10.1128/AEM.71.9.5544-5550.2005
Prescott, C. E., and Grayston, S. J. (2013). Tree species influence on microbial communities in litter and soil: current knowledge and research needs. Forest Ecology and Management, 309, 19-27.
Qiao, J., Liu, Y., Hong, F., and Zhang, J. (2014). A review of catalysts for the electro reduction of carbon dioxide to produce low-carbon fuels. Chemical Society Reviews, 43(2), 631-675.
Rahman. M.H, Bahauddin. M, Khan. M.A.S.A, Islam. M.J, and Uddin. M.B (2012) Assessment of soil physical properties under plantation and deforested sites in a biodiversity conservation area of north-eastern Bangladesh. International journal of environmental sciences volume 3, no 3, ISSN 0976 – 4402.
Roohallah S, Riseh M, Hassanisaadi M,Vatankhah F, S and Rajender S. V ( 2022) Nano/microencapsulation of plant biocontrol agents by chitosan, alginate, and other important biopolymers as a novel strategy for alleviating plant biotic stresses international journal of biological macromolecules.volume222 part A pg 1589-1604
Rousk, J., Bååth, E., Brookes, P. C., Lauber, C. L., Lozupone, C., Caporaso, J. G. and Fierer, N. (2010). Soil bacterial and fungal communities across a pH gradient in an arable soil. The ISME journal, 4(10), 1340.
Schmidt, T. M. and C. Waldron. (2015). Microbial diversity in soils of agricultural landscapes and its relation to ecosystem function. Pages 135-157 in S. K. Hamilton, J. E. Doll, and G. P. Robertson, editors. The Ecology of Agricultural Landscapes: Long-Term Research on the Path to Sustainability. Oxford University Press, New York, New York, USA.
Suzanne L. I (2017) Plant-microbial interactions in agriculture and the use of farming systems to improve diversity and productivity. AIMS Microbiology. 2017; 3(2): 335–353.Published online 2017 May11. doi:10.3934/microbiology.2017.2.335
Umeri C, Onyemekonwu, R.C and Moseri, H (2017) Analysis of Physical and Chemical Properties of Some Selected Soils of Rain Forest Zones of Delta State, Nigeria. Nigeria. Agricultural Resource Technology: Open Access Journal. 5(4): 555668. DOI: 10.19080/ARTOAJ.2017.05.555668
Van Der Heijden, M. G., Bardgett, R. D., and Van Straalen, N. M. (2008). The unseen majority: soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecology letters, 11(3), 296- 310.
Vieira, E.N. (2015); comparison of microbial numbers in soils by using various culture media and temperatures. Journal of microbiological research 160:197-202.
Wardle, D. A. (2006). The influence of biotic interactions on soil biodiversity. Ecology letters, 9(7), 870-886.
Yager, G.O, Agbidye, F.S, and Adma, E, S. (2017) insect species diversity and abundances in and around Federal University of Agriculture, Makudi forestry Nursery. Benue state, Nigeria. Asia journal of biology 4(4)1-11, article no. AJOB 38840 ISSN 2456-7124.
Zhang J, Chen, L Fang L, Jun Y and Shaomin H(2017) Bacterial Community Structure after Long-term Organic and Inorganic Fertilization Reveals Important Associations between Soil Nutrients and Specific Taxa Involved in Nutrient Transformations. Front. Microbiology. 09 February 2017 Sec. Terrestrial Microbiology Volume 8 – 2017 |



