Sisay Argaye; Belachew Bekele*
Ethiopian Institute of Agricultural Research, Holeta Agricultural Research Center, P.O.box 31, Holeta, Ethiopia
| ARTICLE INFO | ABSTRACT |
| Article history: Received: 15.08.2021 Accepted: 16.09.2021 Published: 31.10.2021 *Corresponding author: Belachew Bekele E-mail: bbekele6@gmail.com Keywords: Faba bean Fungicide demonstration Gall Management | Faba bean (Vicia faba L.) is the most important legume crop in Ethiopia and the most single subsistence crop after the staple cereals. However, its production is hampered by various biotic, abiotic, and socio-economic factors. Among the biotic constraints, a newly emerging disease known as Faba bean gall (Olpidium viciae) has become a serious threat to faba bean production in the country. Thus, a field experiment was conducted to demonstrate effective fungicides to manage faba bean gall under natural infection. Two fungicides viz; Noble 25 WP and Ridomil 80 WP were demonstrated alongside the control plot. The result revealed that the use of Noble 25 WP and Ridomil 80 WP reduces fava bean gall explained by reducing AUDPC and percentage severity index (PSI) as well as higher yield. Noble 25 WP recorded the lowest faba bean gall PSI (15%) and AUDPC (855%/days). A higher yield of 58.04% Noble 25 WP and 18.43% Ridomil 80 WP sprayed plots were recorded compared to the control. A marginal rate of return of 681.90% and 678.16% were obtained from plots sprayed with Nobble 25WP and Ridomil 80 WP fungicides. Thus, those fungicides will be used as a component in the integrated management of this disease. |
INTRODUCTION
Faba bean (Vicia faba L.) is grown in many countries as a rain-fed and irrigated crop for human food and animal feed and plays important roles in the national economy and agricultural production in various ways. It is one of the most important pulse crops as it has high yield potential and protein-rich grains and hence serves as human
consumption and animal feed. In many developing countries it is the major source of protein in their feeding culture. Likewise, in Ethiopia, the faba bean is the leading protein source for the rural people and is used to make various traditional dishes (Senayit and Asrat, 1994), which otherwise includes mainly cereals or root crops.
It also plays a fundamental role in sustaining agricultural production and productivity through nitrogen fixation and crop rotation (Torres et al., 2006, Braich et al., 2016). Ethiopia is one of the largest faba bean producers ranked second after China. In highland areas of Ethiopia, faba bean is a major staple food crop among pulses and the most vital legume crop after the staple cereals (Teklay et al., 2018). It is a dominant legume crop in area coverage and production than the other pulses in the country (CSA, 2020). Despite the availability of high-yielding varieties, the average national yield of faba beans is 2.16 tonnes/ha (CSA, 2020).
Its production and productivity are adversely affected by biotic factors (insect pests, parasitic weeds, and mainly foliar diseases), abiotic factors (drought, heat, and acidity), and poor agronomic practices causing a steady reduction in many countries. Among the fungal diseases, chocolate spot (Botrytis fabae), root rots (Fusarium spp.), rust (Uromyces viciae fabae), downy mildew (Peronospora viciae), and Ascochyta blight (Ascochyta fabae) is the most destructive constraints to the crop (Beyene et al., 2016; Maalouf et al., 2019). Diseases are among the most important biotic factors, causing faba bean yield reduction.
More than 17 pathogens have been reported so far on faba beans, from different parts of the country and the most important yield-limiting diseases are chocolate spot (Botrytis fabae), rust (Uromyces vicia fabae), black rot (Fusarium solani), ascochyta blight (Ascochyta fabae) and faba bean necrotic yellows virus (FBNYV) (Dereje and Tesfaye, 1994). Nowadays the crop is threatened by new gall-forming disease (Olpidium spp.) with typical symptoms of, green and sunken on the upper side of the leaf, bulged at the backside of the leaf and finally developing light brownish color lesion, chlorotic galls which progressively broaden to become uneven circular or elliptical spots (Endale et al., 2014) which is causing yield losses as high as 100% (Chala et al., 2017). The pathogen is now becoming a priority biosecurity threat for the production of the crop in the country (Wulita et al., 2019).
The disease was first recorded in Japan in 1912 as faba bean galls (Olpidium viciae Kusano), and also it is a key disease in the highlands of Songpan, Xiaojin, Maekang Sichuan Gansu, Tibet, and Shanxi provinces of China (Xing, 1984; Li-juan et al., 1993). So far, little has been known about Faba bean galls management, and only fungicidal management recommendations have been recommended for the growers. Spraying of Ridomil fungicide reduces the disease severity and gives better yields than other fungicides applied and control plots (Bitew et al., 2016). Applications of Bayleton 25 WP and Ridomil gold reduced disease severity and gave higher yields than control/unsprayed plots (Teklay et al., 2018). Thus, the objectives of this study were to demonstrate effective fungicides for the management of faba bean gall disease.
MATERIALS AND METHODS
This experiment was conducted at Degem (hot spot area for the disease) in the 2019 main cropping season. A local faba bean variety was used to execute the experiment and seed rate was applied as a recommendation in row planting. Two fungicides; Ridomil Gold and Noble 25 MZ were demonstrated. Plot sizes of 10m x 10m with a spacing of 1.5 m between plots were used.
The foliar spray fungicides were applied three times using a knapsack sprayer starting from the first appearance (at the vegetative stage of the crop) of the disease and water was sprayed on control plots. Fungicides were applied as manufacturers’ recommendations. Spraying was applied three times at the time of the disease’s appearance (seedling) and repeated two times before the start of the flowering and podding stage. The date of seedling emergence and the first date of bean gall disease appearance were recorded. Also, disease severity, Plant height, Number of pods per plant, thousand seed weight, and seed yield (grain yield) were recorded.
Data collection
Disease severity:
Disease severity was recorded on 20 randomly selected plants in the two central rows of each plot starting from the onset of the disease and repeated after every 10-day intervals. A 0-9 scale was used where 0 = no disease symptom observed, 1 = < 2% plant parts infected, 2 = 2 – 5% plant parts infected, 3 = 6 – 10% plant parts infected, 4 = 11 – 25% plant parts infected, 5 = 26 – 50% plant parts infected, 6 = 51 – 75% plant parts infected, 7 = 76 – 90% plant parts infected, 8 = 91 – 99% plant parts infected, 9 = 100% plant parts infected (Ding et al., 1993). Disease severity scores were converted into a percentage severity index (PSI) for analysis (Wheeler, 1969).
������ =������
������ �� ������ ��100
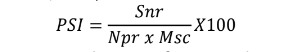
Where Snr is the sum of numerical ratings, Npr is the number of plants rated, and Msc is the maximum score of the scale. Means of the severity from each plot were used in data analysis.
The area under disease progress curve (AUDPC):
AUDPC was calculated for each plot using the formula of Shanner and Finney (1977) and ��−1
expressed in %-dp������ = ∑ 0.5(���� +

Plant height (cm): The height of the plant was measured from the ground to the tip of five randomly taken plants at maturity. The number of pods per plant: The average number of pods per plant was determined from five randomly taken plants.
Hundred seed weight (g): The weight of 100 randomly taken seeds was recorded from each plot. Total grain yield (t h-1): The grain yield per plot was then converted into yield per hectare basis. Percent relative grain yield loss (RYL) was calculated as follows:
������ (%) = (����−����)
������100)
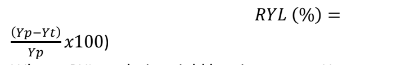
Where, RYL = relative yield loss in percent, Yp = yield from the maximum protected plots and Yt = yield from other plots
Cost-Benefit Analysis
Prices of faba bean seed (Birr ton-1) from a local market and total sales from one hectare were computed. The price of fungicides, labor costs for chemical application, and equipment were also recorded. Partial budgeting was used to assess the profitability of any new technologies to be imposed on the agricultural business (CIMMYT, 1988).
To measure the increase in net return associated with each additional unit of cost (marginal cost), the marginal rate of return (MRR) was calculated as:
MRR =����
ΔIC
1 + ����)(���� + 1 − ����)
��=1
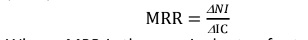
Where, MRR is the marginal rate of returns, Where Xi is the cumulative disease severity at the ith observation; the independent variable ‘x’ indicates the AUDPC level in percentage; is the time (days after planting) at the ith observation and n is the total number of observations.
Crop Parameters
ΔNI – change in net income compared with control, and ΔIC – change in input cost compared with control. The following points were considered during the cost-benefit analysis using a partial budget.
- Costs for all agronomic practices were uniform for all treatments within sites.
- Costs of labor and spray equipment were taken.
- Costs, returns, and benefits were calculated per hectare basis.
RESULTS AND DISCUSSION
Disease severity:
The newly emerged faba bean disease “faba bean gall” was first observed at the vegetative stage at cropping. At the initial assessment dates, fungicides sprayed and unsprayed plots didn’t show variation from each other (Table 1). On the other hand, at the final disease assessment date, fungicide application plots showed high differences in disease severity.
Throughout the season the highest disease severity was recorded from unsprayed control plots. Also, in the last disease recording date (64%) highest final percent severities were recorded from those plots (Table 1). In contrast, Noble 25 WP sprayed plots showed the lowest disease severity (15%) also Ridomil 80 WP sprayed plots recorded lower disease severity (25%) than unsprayed plots.
This finding coincides with the result of (Wulita et al., 2019); and Alemu and Tadele (2017) who reported that foliar-applied fungicides reduced the severity of faba bean galls on faba beans compared to the unsprayed control plots. In general, fungicide application didn’t completely control the development of newly emerged disease “faba bean gall” epidemics but reduced the disease’s severity and the loss incurred by the pathogen.
The area under disease progress curve (AUDPC):
Both demonstrated fungicides showed the lowest AUDPC value than control plots. As control plots recorded the highest disease score throughout the season, the highest (1955%) AUDPC value was recorded followed by (1380%) plots receiving Redomil 80 WP fungicide (Table 2). In contrast, Noble 25 WP sprayed plots recorded the lowest AUDPC (825%) value. Belachew et al., (2018) fungicides have been also recorded as highly effective in reducing the area under the disease progress curve of faba bean gall disease. Control plots recorded the highest disease score throughout the season with an AUDPC value of 1955%.
Plant height: Plant height was shown different among the demonstrated fungicides and control treatments. The shortest (111.0) plant heights were obtained from the unsprayed control plots in the cropping season. On the other hand, the tallest (138.0) plant heights were obtained from plots sprayed with Noble 25 WP fungicide. This result is in line with the finding of DBARC (2015), which reported that faba bean gall diseases significantly reduced the height of the faba bean crop (Table 1).
Numbers of pod per plant: The number of pods per plant showed highly significant variations among the fungicides and the control. The lowest (20.00) and the highest (21.8) mean numbers of pods per plant were obtained from the unsprayed control plots and Ridomil 80 WP sprayed fungicides. Also, the same result, the highest number of pods per plant was recorded on Chlorothalonil and Redomoil sprayed plots (Bitew et al., 2016) (Table 1).
Grain yield and hundred seed weight: Both demonstrated fungicides were given better grain yield and hundred seed weight than unsprayed control plots (Table 1). Grain yield was significantly increased by fungicide sprays. The lowest (1.07 t. ha-1) grain yield was recorded from unsprayed control plots, while the highest (2.55 t. ha-1) yields were obtained from Noble 25 WP sprayed plots. Generally, both fungicide-treated plots gave a higher grain yield than the unsprayed control plot. The highest hundred seed weights of 46.8g followed by 43.0 g were obtained from Noble 25 WP and Ridomil 80 WP fungicides sprayed plots respectively, whereas, the lowest (37.1 g) were recorded from unsprayed control plots.
Table 1. Effect of fungicides on disease severity and yield and yield components of faba bean
| Fungicides | Final DS (%) | PH (cm) | AUDPC% | Pod/plant | Yield ton ha-1 | HSW(g) |
| Noble 25 WP | 15 | 138 | 825 | 20.2 | 2.55 | 46.8 |
| Ridomil 80 WP | 25 | 133 | 1380 | 21.8 | 2.08 | 43.0 |
| Control | 64 | 111 | 1955 | 20 | 1.07 | 37.1 |
DS: Disease Severity, PH: Plant Height, AUDPC: Area Under Disease Progress Curve, HSW: Hundred Seed Weight
Relative Yield Loss in Grain (RYL)
The untreated plot notably recorded the highest relative yield losses (58.04%) and hundred seed weight losses (20.73) (Table 2). On Ridomil 80 WP sprayed plots also recorded 18.43% relative yield loss and 8.12% hundred seed weight losses as compared to plots received Noble 25 WP fungicide as the order mentioned in the 2019/20 cropping season.
Table 2. Effect of fungicide application on yield and hundred seed weight of faba bean
| Fungicides | Yield ton ha-1 | RYL (%) | Hundred Seed Weight (g) | RHSWL (%) |
| Control | 1.07 | 58.04 | 37.1 | 20.73 |
| Ridomil 80 WP | 2.08 | 18.43 | 43.0 | 8.12 |
| Noble 25 WP | 2.55 | 0.00 | 46.8 | 0.00 |
Where; RYL: Relative Yield Loss, RHSWL: Relative Hundred Seed Weight Loss
Cost-benefit Analysis
Results from the assessment of economic returns in this study indicated that fungicide application for faba bean gall disease management demonstration was profitable. Maximum of (ETB 21,826.02) followed by (ETB 18,030.64) net benefits were obtained from plots sprayed Noble 25 WP and Ridomil 80 as compared to unsprayed control plots (ETB 9,892.69) in the 2019 cropping season (Table 3). Beyene and Abiro (2016) also reported application of bayleton and mancozeb fungicides against faba bean gall disease was more profitable than unsprayed control plots. Also, Belachew et al., (2018)
reported that three times spraying of Ridomil fungicide resulted in the maximum marginal rate of returns as compared to unsprayed control plots. Also, Rechcing (1997) stated that fungicides are used because they provide effective and reliable disease control, deliver products in the form of crop yield and quality at an economic price, and can be used safely.
Table 3. Partial budget analysis of fungicide application
| Fungicides | Yield ton ha-1 | Price (ETB ton-1) | Sale revenue | Margina l cost (ETB ha – 1) | Net profit (ETB ton 1) | Marginal benefit (ETB ton-1) | Margina l rate of return (%) |
| Noble 25 WP | 2.55 | 9,245.50 | 23,576.02 | 1,750.00 | 21,826.02 | 11,933.33 | 681.90 |
| Ridomil 80 WP | 2.08 | 9,245.50 | 19,230.64 | 1,200.00 | 18,030.64 | 8,137.95 | 678.16 |
| Control | 1.07 | 9,245.50 | 9,892.69 | 0.00 | 9,892.69 | 0.00 | 0.00 |
CONCLUSION
It was concluded that spraying of Noble 25 WP and Ridomil 80 WP reduced “faba bean gall” disease severity and AUDPC. Application of these fungicides gave higher yield and net profit than control plots. Also, applications of both fungicides were economical and profitable. Thus, it is recommended that these fungicides can be used as one component of disease management. Thus, variety with the supplement of both fungicides with some cultural practices will increase crop production.
ACKNOWLEDGMENTS
The authors are thankful, the Australian Center for International Agricultural Research; Faba Bean in Ethiopia- Mitigating disease constraints to improve productivity and Sustainability project for financing to conduct the activity, and Holleta Agricultural Research Center for facilitating everything to conduct the experiment.
REFERENCES
Alemu, G. Y.; Tadele, Y. A. Management of Faba bean gall disease through the use of host resistance and fungicide foliar spray in northwestern Ethiopia. Adv Crop Sci Tech. 2017.
Belachew, B.; Woubit, D.; Bekele, K.; Selvaraj, T. Management of Faba bean gall disease using cultivars and fungicides in north Showa zone of central Ethiopia. IJRAS. 2018, 5 (1), 6–33.
Beyene, B.; Abiro, T. Management of faba bean gall disease (Kormid) in north Shewa highlands, Ethiopia. ACST, (4), 225 pp.
Beyene, B. Survey and identification of new faba bean disease (Qormid) in the highlands of North Shewa, Ethiopia. CRMB, 2016, 3(1), 561-563.
Braich, S.; Sudheesh, J.; Paull.; Kaur, S. Construction of genetic linkage map and QTLs identification for Ascochyta blight (AB) resistance and flowering time in Faba bean (Vicia faba L.), in Proceedings of the Australian pulse conference, Tamworth, Australia, September 2016.
Chala, D.; Abraham, N.; Zerihun, A.; Meseret, T. Assessment of the occurrence and prevalence of Faba bean gall (Olpidium viciae) in Western highlands of Oromiya, Ethiopia. JNSR. 2017, 7(5), 63–67.
CIMMYT (International Maize and Wheat Improvement Center). From agronomic data to farmers’ recommendations: Economic training manual. 1988, l, 79.
Central Statistical Agency report on area and production of major crops (private peasant holdings, meher season). Statistical Bulletin. 2020, 1, 10–17.
Dereje, G.; Tesfaye, B. Faba bean disease in Ethiopia. In. Asfaw Telaye.; Geletu Bejiga; Mohan C.; Saxena; Mahmoud, B (Eds.), cool-season food Legumes of Ethiopia. Proceedings of First National Cool-season Food Legume Review Conference, Addis Ababa, Ethiopia, December 16-20, 1994, ICARDA/IAR.ICARDA, 328-345.
Ding, G.; Xung, L.; Oifang, G.; Pingxi, L.; Dazaho, Y.; Ronghai, H. Evaluation and screening of Faba bean germplasm in China. FN. 1992, 32, 8–10.
Endale, H.; Gezahegne, G.; Tadesse, S.; Negussie, T.; Beyene, B.; Anteneh, B.; Daniel, K.; Tamene, T. Faba Bean Gall; a New Threat for Faba Bean (Vicia faba) Production in Ethiopia. ACST, 2014, 2, 144.
Li-juan, L.; Zhao-hai, Y.; Zhao-jie, Z.; Ming-shi, X.; Han-qing, Y. Faba Bean in China: State-of-the-art Review Special Study Report (English Translation). International Center for Agricultural Research in the Dry Areas (ICARDA). 1993, P.O. Box 5466, Aleppo, Syria.
Maalouf, F.; J. Hu, D. M.; O’Sullivan. “Breeding and genomics status in faba bean (Vicia faba),” Plant Breeding. 2019, 138(4), 465–473.
Rechcing, N. A.; Rechcing, J. E. Environmentally safe approaches to Crop disease control. CRC. Lewis Publisher, New York, 1997.
Senayit, Y.; Asrat, W. Utilization of cool season food legumes in Ethiopia. In: Asfaw, T.; Saxena, M.; Solh, M.; Geletu, B. (eds.). Cool season food legumes of Ethiopia. 16-20 December 1993, Addis Ababa, Ethiopia. ICARDA/Institute of Agricultural Research, 1994.
Shanner, G. Finney R. Inheritance of slow mildewing resistance in wheat proceedings. American Phytopathological Society. 1977, 2, 49.
Teklay, A.; Gurja, B.; Gemechu, K.; Taye, T. Fungicidal management of the newly emerging Faba bean disease gall (Olpidium viciae Kusano) in Tigray, Ethiopia. Crop protection. 2018, 107, 19–25.
Torres, M.; Roman, B.; Avila, M. Faba bean breeding for resistance against biotic stresses: towards application of marker technology. Euphytica, 2006, 147(1-2), 67–80.
Wheeler, E. J. An Introduction to plant diseases. Wiley and Sons, London. 1969, 374 pp.
Wulita, W.; Mashila, D.; Nigussie, T.; Seid, A. Fungicide management of Faba bean gall (Olpidium viciae ) in Ethiopia. TJAFST. 2019, 7(7), 1075–1081.
Xing, Z. Faba bean gall disease caused by Olipidium and its control. Acta Phytopathological Sinica. 1984, 14 (3), 165-173.



