Volume no.2, Issue no. 4, Year 2023 www.sarpo.net
Djeugap F.J.1*, Nouteka K.J.N.1, Ngalle T. S.1, Dida L.S.L.2, Essomo E.S1, Keegoui G.1, Kuenbou M.J.1, Abireche U.H.1, Galani Y.H.J.4
1Phytopathology and Agricultural Zoology Research Unit, Department of Crop Sciences, Faculty of Agronomy and Agricultural Sciences, University of Dschang, Box. 222 Dschang, Cameroon.
2Phytopathology and Plant Protection Research Unit, Department of Plant Biology, University of Yaoundé 1, Box 812, Yaoundé-Cameroon.
3Applied organic chemistry Research Unit, Department of Chemistry, Faculty of Science, University of Dschang, Box 67, Dschang, Cameroon.
4Section of Natural and Applied Sciences, School of Psychology and Life Sciences, Canterbury Christ Church University, Canterbury, CT1 1QU, UK
| ARTICLE INFORMATION | ABSTRACT | |
|---|---|---|
| Corresponding author: Djeugap F.J. E-mail: jdjeugapfovo@yahoo.fr Keywords: Seed dormancy Plant extracts Seed-borne fungi Tetrapleura tetraptera Edible non-timber forest products Received: 29.07.2023 Received in revised form: 03.08.2023 Accepted: 12.08.2023 Publication date: 24.10.2023 | Seed dormancy and seed-borne fungi are main constraints to the domestication of edible non-timber forest products such as Tetrapleura tetraptera. This study aimed to evaluate the effect of thermal and mechanical scarification on seed dormancy and the efficacy of four plant aqueous extracts (Cymbopogon citratus, Callistemom viminalis, Tephrosia vogelii, Eucalyptus saligna) against seed-borne diseases and seedlings vigour of T. tetraptera. Treatments consisted of soaking the seeds in water at 70°C for 2 and 4 hours; and seed scarification with abrasive paper at 1 mm and 2 mm depths. The biological activity of plant extracts was evaluated both in vitro (by the dispersion method on agar medium) and in vivo at 0.25, 0.50 and 0.75 mg/mL. Momtaz (Imidacloprid 250 g/kg + Thiram 200 g/kg) was used as a positive control. Dormancy was lifted by scarification at 2 mm depth; this treatment had the highest germination percentage (92.33%) and the lowest infection rate(20.67%). The more frequent seed-borne fungi isolated belong to Aspergillus spp. (18.43-21.78%). The pathogenicity test was positive with Alternaria alternata, A. fumigatus and Cercospora sp. T. vogelii extract totally inhibitedthe growth of the pathogenic fungi at all the concentrations tested. Seeds infection with C. viminalis (5.33%) and T. vogelii (4.12%) extracts at 1 mg/mL were significantly similar to Momtaz (3.33%). The extract of C. viminalis had the highest vigour index (674.42) at 0.75 mg/ml. Mechanical scarification using abrasive paper and seeds treatment with plant extracts of T. vogelii could be used in the domestication process of the species. |
INTRODUCTION
Forests provide essential goods and services for food, health, and income for 1.2 billion people worldwide (Betti et al. 2016). Among these resources, edible non-timber forest products (ENTFP) occupy a prominent place correlated with their food, socio-economic, and cultural values, which are very decisive for raising the standard of living of the populations (Manfo, 2018). NTFPs constitute a reserve or safety net, a source of subsistence and income in the event of crop losses, shortages, unemployment, or other emergencies or difficulties resulting from disasters or calamities (Muyambo et al. 2017). However, production, conservation, and processing techniques are still rudimentary, and parasitic attacks are significant on ENTFP (Awono et al. 2016). These attacks cause many losses in the production and conservation of ENTFP and considerably reduce the contribution of the ENTFP sector in the fight against food insecurity. Among the ENTFP of tropical Africa, Tetrapleura tetraptera is a perennial Mimosaceae tree that reaches 35 to 50 cm in diameter at maturity and bears buttresses at the base (Eyog et al. 2006). Its fruits have four protruding dark green sides when they are formed and become dark brown and shiny when ripe. The ripe fruits of T. tetraptera are dried and used as spice condiments, as well as for their medicinal properties in the treatment of digestive diseases, cysts, myomas, and obesity (OAU/STRC, 1996). The result of a study conducted by the Tropenbos Cameroon program in 2017 in southern Cameroon reveals that a cut pile of T. tetraptera costs between FCFA 50 and 200 (USD 0.08 to 0.33) and that a pod is sold between FCFA 150 and 300 (USD 0.25 to 0.50) per unit (Walter, 2001). This spice is also exported and sold in the European markets (Sunderland, 2000). In 2000, the United Kingdom imported 20 tonnes from Nigeria, Ghana, and Cameroon (Tabuna, 2000). Despite this socio-economic importance, the production of T. tetraptera faces several difficulties including difficult seed germination and high seed infection of seed-associated fungi (Ndoye et al. 2000). In fact, because of the rigidity of the pod and the seed coat, and because of the un-permeability of the seed to water, it remains in a state of dormancy. This makes the natural germination of T. tetraptera almost impossible without the disseminator action of elephants or people (Alexandre, 1978). To overcome this constraint, various techniques have been applied to T. tetraptera seeds to lift the dormancy. Wakaya and Akinyele (2016) used 98% concentrated sulfuric acid, lime juice, overnight soaking, and hot water to stimulate T. tetraptera germination and found that dormancy can be broken by sulfuric acid. However, this technique has proven difficult to apply, and not accessible to low-income producers. The establishment of techniques within the reach of producers and easily applicable is, therefore, necessary to efficiently germinate the seed of T. tetraptera. To manage these fungal diseases of seeds (damping off, seed rot, fusarium, etc.) whose pathogens are mostly telluric (Rhizoctonia, Cercospora, Aspergillus, Colletotrichum, Fusarium, etc.), several synthetic fungicides are recommended. Unfortunately, their judicious use in accordance with the prescriptions of use is rather rare because the local nursery workers are inexperienced and untrained in the management of pesticides. The sometimes anarchic use of synthetic pesticides presents risks of environmental pollution, development of resistance to pathogens, and intoxication for the user (Lulia et al. 2012; Kausik and Sayan, 2015). For these reasons, research in the field of plant protection is increasingly oriented towards the development of alternatives to chemical control by resorting to the use of non-toxic substances of natural origin (Murray, 2000; CABI, 2011). Hence, this study was carried out to evaluate the effect of thermal and mechanical scarification on seed dormancy and the effectiveness of four plant extracts against seed pathologies and seedling vigour of T. tetraptera.
MATERIAL AND METHODS
Collection and Conservation of Plant Material
The fruits of T. tetraptera were picked under five trees about 20 years old in the locality of Kekem, in the West region of Cameroon. These fruits were dried at room temperature in the laboratory for two months before the test. Using a hammer, the fruits were carefully broken to obtain the seeds which were stored in sterilized and hermetically sealed glass boxes. The leaves of the plants used for the preparation of the extracts, namely Eucalyptus saligna, Tephrosia vogelii, Callistemon viminalis and Cymbopogom citratus, were harvested from 6 – 8 a.m. at the Application and Research Farm of the Faculty of Agronomy and Agricultural Sciences (FASA) of the main campus of the University of Dschang in the West region of Cameroon.
Scarification of Tetrapleura tetraptera Seeds
The seeds were disinfected in a 2% hypochlorite solution for 3 min, then rinsed 3 times with distilled water for 5, 10 and 15 minutes respectively to remove traces of the disinfectant. The disinfected seeds were then scarified either by heat infusion of the seeds in water at 70°C for 2 hours or for 4 hours; either mechanically with abrasive paper by rubbing the proximal part of the seed (hilum) to a depth of 1 mm or 2 mm. The control batches consisted of seeds that had received no treatment. The sacrificed seeds were then placed in 15 cm-diameter glass Petri dishes previously lined with three layers of blotting paper soaked in water and then incubated at 22 ± 1°C under a 12 h of light and 12 h of darkness photoperiod, and watered every other day. There were 100 seeds per treatment and each treatment was repeated 3 times. The date of first germination, germination rate and seed infection rate were assessed daily. The Germination (G) was calculated as follows: G = g x 100/N, where g = number of seeds germinated and N = total number of seeds sowing (Djeugap et al., 2014). The infection rate (IR) reflects the susceptibility of seeds to fungal infections during the germination period: IR = i x 100 /N with i = number of seeds infected and N = total number of seeds sowing. The germination rate (GR) was calculated through the relationship GR = ∑n / ∑ (n x DAS) with n = number of seeds germinated on day d and DAS = the number of days after sowing.
Isolation and Identification of Seed-borne Fungi of Tetrapleura tetraptera
The medium used for the culture of fungi was potato dextrose agar (PDA) supplemented with 1 g/L of chloramphenicol and sterilized at 121 °C for 15 min. Tetrapleura tetraptera seeds, whether symptomatic or not, were disinfected in a 3% hypochlorite solution for 2 min and then rinsed with sterile distilled water (Djeugap et al. 2015). Ten disinfected seeds were aseptically deposited in 90 mm Petri dishes containing 20 ml of PDA medium and placed in an incubator at 22°C. The fungal colonies visible around the inoculated seeds observed 5 days after inoculation, were then purified on the PDA medium (Djeugap et al. 2017). The isolation frequency (IF) of each fungus was calculated using the following formula: IF = (NF/NT) x 100 where NF represents the total number of samples from which a particular fungus was isolated and NT, the total number of samples from which the isolations were made (Iqbal and Saeed, 2012). The identification was made on the basis of the morphological characteristics of the purified fungi (mycelium and fructification) as observed under the microscope using the identification keys of Mycology (Champion, 1997).
Pathogenicity Test
For each 10-day-old isolated fungus, a spore suspension was prepared for inoculation by adding 10 ml of sterilized distilled water to the Petri dishes containing the PDA culture medium, and then gently rubbing with a thin brush. A drop of Tween 80 was added to homogenize the spores in the suspension, then the suspension was filtered using a filter cloth (mesh diameter < 1mm) to remove the mycelial fragments (Imathiu et al. 2014). Finally, 10μl of each suspension was deposited on a hemacymeter (Thoma cell) and mounted on a microscope to count the number of spores and subsequently determine the concentration of the suspension through the formula of Mathur and Kongsda (2003): (Number of spores x volume of spore suspension in mL)/Counting area in mm² x depth of counting area in mm)/1000. This number of spores was calibrated at 2.4 x 105 spores/ml for all the fungal species used for the pathogenicity test. The seeds disinfected as indicated above (Djeugap et al. 2015) were placed in Petri dishes containing 3 layers of sterilized blotting paper and moistened with sterile distilled water at the rate of 100 seeds per box. Then, a 10 ml spore suspension of each fungus was sprinkled on these seeds and the dishes were finally sealed with parafilm and incubated at room temperature (22±1°C). Each treatment was repeated 3 times and the seed infection rate was determined.
Preparation of Plant Extracts
Fresh leaves harvested from the plants were washed with tap water to remove microorganisms and dust and then dried in the shade for two weeks. The dried leaves were ground to obtain a fine powder. To obtain aqueous extracts, 100 g of the plant powder was macerated with 500 ml of sterile distilled water for the aqueous extract, for 24h with two shaking, in the dark. The mixture was filtered using muslin, cotton, and Whatman No. 4 paper and the filtrate constituted the crude plant extract (Falleh et al. 2008).
Evaluation of the Antifungal Potential of the Plant Extracts
The evaluation of the in vitro activity of the extracts was carried out following the dispersion method on agar medium (PDA) at concentrations of 2.5, 5.0, and 7.5 mg/ml on the three fungi which were positive in the pathogenesis test namely: Alternaria alternata, Aspergillus fumigatus and Cercospora sp. Sterilized distilled water and the synthetic fungicide Momtaz (Imidacloprid 250 g/kg + Thiram 200 g/kg) served as a negative and positive control, respectively. The radial growth (RG) of the pathogen was evaluated by the formula: RG= (d1+d2- 2d0) / 2 by measuring the growth diameters every day from two orthogonal lines drawn on the back of the boxes and crossing at the level from the point of deposition of the explant. In this formula, d0 is the diameter of the explant, and d1 and d2 are the two measured orthogonal diameters of the culture. The percentages of inhibition (%I) were determined by the relationship %I = 100 (Dc – Df) / Dc where Dc is the growth diameter of the control and Df is the growth diameter of the fungal colony on medium supplemented with extracts (Yaouba et al. 2017).
Evaluation of the Efficacy of Plant Extracts on Seed Germination and Infection, and Vigour Index of Seedlings of Tetrapleura tetraptera
Seeds scarified and disinfected (200 seeds each were considered per treatment) were soaked with 25 ml of aqueous extracts at concentrations of 0.25, 0.5, 0.75 and 1.0 mg/ml. A volume of 25 ml of sterilized distilled water and Momtaz fungicide mixture (Imidacloprid 250 g/kg + Thiram 200 g/kg) at 5 mg/kg respectively served as negative (T-) and positive (T+) controls. After soaking, the seeds were placed in an oven at 40°C for 15 min following the modified protocol of Djeugap (2013) then inoculated in Petri dishes layered with moist paper. The Petri dishes containing the seeds were stored at room temperature with a 12 h of light and 12 h of darkness photoperiod. Each treatment was repeated 3 times. The daily observations carried out focused on counting the number of germinated seeds and the number of healthy seeds in order to calculate the infection rate and the seed germination rate. The seedling vigour index was obtained as follows. Vigour Index = Germination Rate x (Root length + Stem length) (Abdul-Baki and Anderson 1973).
Statistical Analysis
The analyses were carried out using the statistical analysis software R version 3.5.1 at the 5% probability threshold. Since the data did not follow the normal distribution and the homogeneity of the variance was not respected, the Kruskal-Wallis test was used for the separation of the means.
RESULTS AND DISCUSSION
Effect of Scarification on Germination and Infection of T. tetraptera seeds
Thermal and mechanical scarification significantly increased the germination rates of T. tetraptera seeds compared to the control where the germination rate was zero (Table 1). The germination rate was maximum with the mechanical scarification at 2 mm depth (92.33%) followed by the mechanical scarification treatment at 1 mm (81.52%), while thermal scarification treatments for 2 hours and thermal scarification for 4 hours showed very low with germination rates of 1.27% and 0.67% respectively. There was no significant difference between the infection rates obtained from the different treatments, but the seed infection rate was higher in the heat scarification treatment boxes for 2 hours (42.00%). The control boxes had no germinated seeds, but a fairly high infection rate (28.67%). Figure 1 shows the germinated and infected seeds at 3 DAS where visible mycelium of the pathogens is observed on seeds. The different results between thermal and mechanical scarification could be explained by the fact that at 2 mm the embryo is already visible and develops without braking due to the integument. Nkongmeneck et al. (1996) showed that scarification using a nail clipper could increase germination capacity by 80 to 90% after three days. The dormancy breaking methods used have been evidenced by other studies, namely Pérez-Garcia & Gonzalez (2006) and Rao et al. (2006) for mechanical scarification which consists of using sandpaper; and Li et al. (1999) for thermal scarification with hot water (80°C) which made it possible to remove the waxy cuticle from the seeds. Some authors highlighted the interest in involving local populations in the domestication and in situ and ex-situ conservation strategies of plant species of interest (Harivel et al. 2006; Meunier et al. 2010).
Effect of Treatments on the Date of First Germination and the Germination Rate
The first germination was observed in the thermal scarification treatment boxes for 4 hours and in the mechanical scarification treatment boxes at 2 mm with a duration of 2.33 and 2.67 days respectively (Table 2). These dates differed significantly from those obtained in the boxes with mechanical scarification treatments at 1 mm and thermal scarification for 2 hours (4.91 and 4.67 respectively). The germination speed was higher in the mechanical scarification treatment boxes (19.85 and 14.10 respectively at 2 mm and 1 mm) unlike the speeds obtained in the thermal scarification treatment boxes (0.10 and 0.27 respectively for 2h and 4h).
Table 1. Germination and infection rate of Tetrapleura tetraptera seeds submitted to different scarification techniques, 14 days after sowing.
| Treatment | Germination(%) | Infection rate(%) |
| Control | 0.0±0.0c | 28.67±1.98b |
| Thermal scarification for2h | 0.67±0.58c | 42.00±4.88a |
| Thermal scarification for4h | 1.23±0.63c | 27.67±2.81b |
| Mechanical scarification at1 mm | 81.52±3.42b | 23.81±4.84c |
| Mechanical scarification at2 mm | 92.33±4.73a | 20.67±5.86c |
a,b,c Data are means ± standard deviations. Means with different superscript letters in the same column are significantly different as per the Kruskal-Wallis test at p= 0.05.
Table 2. Effect of scarification on the germination date and germination rate of de Tetrapleura tetraptera seeds at 14 days after sowing.
| Day of the first germination | Germination rate (seeds/day) | Germinationrate (seeds/day) |
| Control | 0.0±00c* | 0.0±00d |
| Thermalscarification for 2h | 4.67±1.16a | 0.10±0.10c |
| Thermalscarification for 4h | 2.33±0.04b | 0.27±0.14c |
| Mechanical scarification at 1mm | 4.91±1.19a | 14.10±2.19b |
| Mechanicalscarification at 2 mm | 2.67±0.58b | 19.85±1.59a |
*Data are means ± standard deviations. Means with different superscript letters in the same column are significantly different as per the Kruskal-Wallis test at p= 0.05.
Seed-borne Fungi of Tetrapleura tetraptera Seeds and their Pathogenicity
Seven species of seed-borne fungi were been identified: Rhizoctonia sp., Aspergillus niger, Aspergillus flavus, Aspergilus fumigatus, Trichoderma sp., Alternaria alternata, Cercospora sp. and Pestalotiopsis sp. The more frequent species isolated were: A. flavus (21.78%), A. niger (18.48%) and A. fumigatus (16.31%); Pestalotiopsis sp had the lowest isolation frequency (Figure 2). The pathogenicity test was positive for A. alternata, A. fumigatus and Cercospora sp. with infection rates of 45.75%, 32.25% and 22.12%, respectively, 5 days after inoculation (Table 3). The seed’s response to artificial inoculation shows a rapid death of seed by these three seed-borne pathogens within 5 days (Figure 3). The isolated fungal species are well known as damaging species of ENTFP seeds. Most of these species have already been identified on Ricinodendron heudelotii and Garcinia kola, two ENTFP of high socio-economic value in Cameroon, as being responsible for the seed losses (Dongmo et al. 2017). Also, Djeugap et al. (2017) reported species like A. flavus and Cercospora sp. as fungi responsible for post-harvest losses of Monodora myristica. Most of these fungi are responsible for the high post-harvest losses of some fruits (Onuorah and Orji, 2015). Cercospora sp. was also isolated from seeds of Persea americana with high occurrence frequencies (Erute and Oyibo, 2008). The high frequencies of certain species such as Aspergillus in seeds of T. tetraptera could be explained by the fact that they are extremely polyphagous and likely to live on more diverse media than other species (Agrios, 2005). Poor storage conditions may also explain their presence in T. tetraptera grains. Some of the fungi inoculated on the grains of T. tetraptera developed and others did not; they latter can be considered as opportunists.
Bio-efficacy of Plant Extracts on the Inhibition Percentage of Pathogenic Fungi of Tetrapleura tetraptera seeds
The extract of C. viminalis at a concentration of 0.75 mg/mL significantly reduced the radial growth of A. alternata and Cercospora sp. by 80.89% and 69.08%, respectively. The extract of C. citratus at the concentration of 0.75 mg/ml was significantly reduced by 60.25%, 41.77%, and 19.49% respectively, the radial growth of A. alternata and Cercospora sp., and A. fumigatus. The extract of Eucalyptus saligna totally inhibited the growth of A. fumigatus at the concentrations of 0.75 mg/mL. The radial growth inhibition was absent in the negative control dishes for all the fungi, while in the positive control dishes enriched with Momtaz, the radial growth of the fungi was completely inhibited (Table 3). Tephrosia vogelii extract totally inhibited the development of the three fungi at all the concentrations tested. The effectiveness of aqueous extracts of T. vogelii and C. viminalis on the development of these microorganisms varies according to concentrations and has been demonstrated by Salem et al., (2017) on C. viminalis and Masete (2021) on T. vogelii. Other studies showed that C. citratus and T. vogelii extracts exhibited antifungal activity against potato late blight pathogen Phytophthora infestans (Galani et al. 2013).

Figure 1. Scarified and germinated seeds of Tetrapleura tetraptera at 3 days (A) and 7 days after sowing (B) in the control (non-treated).
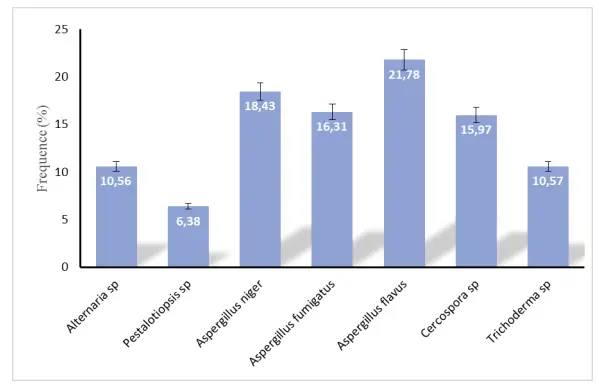
Figure 2. Occurrence/frequency of fungi isolated from Tetrapleura tetraptera seeds.

Figure 3. Pictures showing seed infection after inoculation with Alternaria alternata (left), Aspergillus fumigatus (center) Cercopora sp. (right), 5 days after inoculation.
Effect of Plant Extracts on Germination, Seed Infection, and Seedlings Vigour of Tetrapleura tetraptera
T. vogelli and C. viminalis extracts gave the highest germination percentage at 0.25 mg/mL (97.33%) and 0.5 mg/mL (96.67%), respectively. However, there was no significant difference between the different concentrations of the extracts and the two controls on the germination of T. tetraptera seeds (Table 5). Also, the lowest seed infection was obtained with the same extracts and the values obtained were significantly (p<0.05) comparable to the positive control (synthetic fungicide). In fact, seed infection with C. viminalis extract was 5.33% at 1 mg/mL and 4.12 % with T. vogelii at the same concentration while in the positive control, it was 3.33%. Significant differences were observed between the different concentrations of the aqueous extracts and the two controls on the percentage of infection of the seeds of T. tetraptera (Table 5). Table 6 shows that the maximum vigour index (674) was obtained with C. viminalis at 0.75 mg/mL while the positive control obtained the lowest vigour index (506). However, significant differences were observed between the different doses of aqueous extracts and the two controls on the vigour index of T. tetraptera seeds.
Table 3. Pathogenicity and infection rate of seed-borne fungi isolated from Tetrapleura tetraptera, 5 days after inoculation.
| Fungi | Pathogenicity | Infection rate (%) |
| Alternaria alternata | +++ | 45.75±4.65a* |
| Pestalotiopsis sp | – | 0.0±0.0d |
| Aspergillus niger | – | 0.0±0.0d |
| Aspergillus fumigatus | ++ | 32.25±2.88b |
| Aspergillus flavus | – | 0.0±0.0d |
| Cercospora sp | + | 22.12±3.66c |
| Trichoderma sp | – | 0.0±0.0d |
*Data are means ± standard deviations. Means with different superscript letters in the same column are significantly different as per the Kruskal-Wallis test at p= 0.05. +++ = highly pathogenic; ++ = moderately pathogenic; + = pathogenic; – = not pathogenic.
The T. vogelii extracts protect the seeds against fungal attack more than the others, followed by C. viminalis,
E. saligna and C. citratus extracts. This activity of the aqueous extracts could be due to their chemical
composition in antifungal substances. These results corroborate those of Yaouba et al. (2019) that showed the antifungal potential of E. saligna extracts against the fungi responsible for the deterioration of green
beans post-harvest; and those of Djeugap et al. (2011) who demonstrated the in vitro and in vivo efficacy of plant extracts including C. viminalis against black nightshade downy mildew; Helal et al. (2017) who showed that C. citratus extract caused inhibition of Aspergillus sp.; and Kpatinvoh et al. (2007) findings showed an inhibitory activity of T. vogelii on the radial growth of fungi in stored cowpea seeds.. This suggests
that the extracts of these plants would be effective in the fight against fungi associated with ENTFP seeds. The antifungal activity of these plant extracts could be due to the action of oxygenated monoterpenes (Yoshimura et al., 2010) and phenolic compounds including sterols, flavonoids, condensed tannins, coumarins and alkaloids (Galani et al. 2013).
Table 4. Inhibition of the mycelial growth of seed-borne fungi of Tetrapleura tetraptera by plant aqueous extracts.
| Plant extracts | Concentration (mg/mL) | Growth inhibition (%) Alternaria Cercospora Aspergillusalternata sp. fumigatus |
| 0.25 | 27.67±3.76c* 18.07±2.33c 0.0±0.0d | |
| 0.50 | 73.33±6.55bc 67.07±4.87bc 0.0±0.0d | |
| Callistemom viminalis | 0.75 | 80.91±11.32bc 69.08±15.26bc 0.0±0.0d |
| 0.25 | 0.0±0.0d 0.0±0.0d 62.65±18.78b | |
| 0.50 | 0.0±0.0d 0.0±0.0d 28.11±6.77bc | |
| Cymbopogom citratus | 0.75 | 60.25±12.41b* 41.77±13.27b* 19.49±5.64c |
| 0.25 | 85.16±9.95ab 77.10±8.29ab 54.21±16.23 b | |
| 0.50 | 82.36±13.11ab 78.31±11,34ab 79.27±20,44ab | |
| Eucalyptus saligna | 0.75 | 95.66±12.78ab 84.34±10,94ab 100±0.0a |
| 0.25 | 100±0.0a 100±0.0a 100±0.0a | |
| 0.50 | 100±0.0a 100 ±0.0a 100±0.0a | |
| Tephrosia vogelii | 0.75 | 100±0.0a 100±0.0a 100±0.0a |
| Momtaz (synthetic | 100±0.0a 100±0.0a 100±0.0a | |
| fungicide) | ||
| Control (distilled water) | 0.0±0.0d 0.0±0.0d 0.0±0.0d |
*Data are means ± standard deviations. Means with different superscript letters in the same column are significantly different as per the Kruskal-Wallis test at 5%.
Table 5. Influence of plant aqueous extracts on the germination (%) of Tetrapleura tetraptera seeds.
| Concentration (mg/ml) | Cymbopogomcitratus | Callistemonviminalis | Eucalyptus saligna | Tephrosia vogelii |
| 0.25 | 86.67±5.77a* | 90.00±7.32a | 86.67±5.27a | 97.33±5.77a |
| 0.50 | 90.00±7.00a | 96.67±5.77a | 84.67±5.77a | 83.33±5.27a |
| 0.75 | 92.00±7.00a | 90.00±7.32a | 80.33±5.27ab | 80.33±6.54a |
| 1.00 | 86.67±5.27a | 80.00±4.10b | 76.67±5.27c | 76.67±5.27b |
| Momtaz (fungicide) | 94.67±5.77a | 94.67±5.77a | 94.67±5.77a | 94.67±5.77a |
| Distilled water (control) | 92.33±11.54a | 92.33±5.54a | 92.33±6.54a | 92.33±6.54a |
*Data are means ± standard deviations. Means with different superscript letters in the same column are significantly different as per the Kruskal-Wallis test at 5%.
Table 6. Effect of plant aqueous extracts on seeds infection (%) of Tetrapleura tetraptera during germination.
| Concentration (mg/mL) | Cymbopogom citratus | Callistemon viminalis | Eucalyptus saligna | Tephrosia vogelii |
| 0.25 | 19.33±5.77b | 20.03±2.07b | 25.63±17.66b | 23.33±15.27b |
| 0.50 | 13.91±6.27b | 12.04±7.55c | 19.33±5.77bc | 16.67±9.77b |
| 0.75 | 12.33±5.77b | 10.22±7.32c | 16.67±5.77b | 4.54±1.96c |
| 1.00 | 8.67±4.01b | 5.33±2.82c | 13.33±7.27bc | 4.12±1.77c |
| Momtaz (fungicide) | 3.33±1.27c* | 3.33±1.27c | 3.33±1.27c | 3.33±1.27c |
| Distilled water(control) | 86.67±15.27a | 86.67±15.27a | 86.67±15.27a | 86.67±15.27a |
(a-c) Data are means ± standard deviations. Means with different superscript letters in the same column are significantly different as per the Kruskal-Wallis test at p= 0.05.
Tableau 7. Effect of aqueous plant extracts on the vigour index of Tetrapleura tetraptera seedlings.
| Concentrations(mg/mL) | Cymbopogomcitratus | Callistemonviminalis | Eucalyptussaligna | Tephrosia Vogelii |
| 0.25 | 541.94±10.86b* | 553.00±14.01b | 410.13±9.28d | 603.43±11.92a |
| 0.50 | 446.96±15.73d | 464.69±36.99c | 533.99±6.78c | 467.23±4.11dc |
| 0.75 | 634.27±7.84a | 674.42±11.25a | 562.44±6.05b | 530.33±7.52b |
| 1.00 | 448.60±10.66d | 388.74±6.30ab | 597.02±12.37a | 432.61±5.04d |
| Momtaz (fungicide) | 506.74±10.95c | 506.74±10.95bc | 506.74±10.95c | 506.74±10.95c |
| Distilled water (control) | 238.31±7.57e | 238.31±7.57d | 238.31±7.57e | 238.31±7.57e |
*Data are means ± standard deviations. Means with different superscript letters in the same column are significantly different as per the Kruskal-Wallis test at p= 0.05.
CONCLUSION ACKNOWLEDGMENTS
This work investigates effective and easily applicable solutions to the dormancy and therefore to the germination of T. tetraptera seeds and proposes a biological control measure for seed-borne fungi. Scarification through mechanical abrasive at 2 mm depth improves the germination rate by up to 92%. The most frequently identified fungi on T. tetraptera seeds were: A. flavus, A. niger and A. fumigatus which may be mycotoxigenic. The pathogenic fungi were: A. alternata, A. fumigatus and Cercospora sp. Aqueous extracts of T. vogelii, E. saligna, C. viminalis, and C. citratum exhibited higher antifungal properties and inhibited the growth of the three pathogenic fungi as well as the synthetic fungicide Momtaz. These plant extracts promoted germination, vigour and seed protection. Since key domestication constraints of T. tetraptera have been underpinned and seed-borne fungi have been identified and controlled by this work, studies on the cultivation of T. tetraptera in the nursery and in the field should now be emphasized.
The farmers from the Kekem sub-division (high Plateau agroecological zone) provided fruits and seeds of T. tetraptera. This study was financed by the authors.
REFERENCES
Abdul-Baki, A.A.; Anderson, J.D. Physiological and biological deterioration of seed. Seed Biology, 1973, 13, 630-633.
Agrios, G. N. Plant Pathology, 5th edition, Elsevier-Academic Press, Amsterdam, 2005; pp 82-102.
Alexandre, D.Y. Le rôle disséminateur des éléphants en forêt de tai, Côte-d’Ivoire. La Terre et la Vie, 1978, 32, 42-72.
Amadi, J.E.; Nwaokike, P.; Olahan, G.S.; Garuba, T. Isolation and identification of fungi involved in the post-harvest spoilage of guava (Psidium guajava) in Awka metropolis. Int. J. Eng. App. Sc., 2014, 4(10), 7-12.
Awono, A. Eba’a, R.A.; Foundjem-Tita, D.; Levang, P. Vegetal non-timber forest products in Cameroon, contribution to the national economy. International Forestry Review, 2016, 18(1), 66-77.
Betti, J.L.; Ngankoué, M.C.; Dibong, S.D.; Singa E.A. Etude ethnobotanique des plantes alimentaires spontanées vendues dans les marchés de Yaoundé, Cameroun. International Journal of Biological and Chemical Sciences, 2016, 10(4), 1678-1693.
Bewley, D.J. Seed germination and dormancy. The Plant Cell, 1997, 9, 1055-1066.
CABI. Natural products in plant pest management. Nawal K. Dubey (Ed.). Centre for Advanced Studies in Botany Banaras Hindu University, Varanasi, India, 2011, 306p.
Champion, R. Identifier les champignons transmis par les semences. Techniques et pratiques. INRA, Editions Quae, Paris France, 1997, 398p.
Djeugap, F.J. ; Fontem, D.A. ; Tapondjou, A.L. Efficacité in vitro des extraits de plantes contre le mildiou (Phytophthora infestans) de la morelle noire. International Journal of Agriculture and Biosciences, 2011, 5(6), 2205-2213.
Djeugap, F.J.; Nzuta, C.; Temgoua, L.F.; Kenmogne, G.; Tekam, P.M. Champignons pathogènes associés aux semences de Pericopsis elata et effet des substrats sur la germination, la croissance et l’infection des plantules au Cameroun. Revue Scientifique et Technique Forêt et Environnement du Bassin du Congo, 2017, 8, 19-27.
Djeugap, F.J. Les contraintes phytosanitaires observées sur les produits forestiers non ligneux d’importance socioéconomique au Cameroun. Rapport d’enquête de terrain effectuée dans les forêts et les marchés locaux des zones forestières du Cameroun. Faculté d’Agronomie et des Sciences Agricoles, Université de Dschang, 2011, 23p.
Djeugap, F.J.; Tsopmbeng, N.G.; Keuete, K.E.; Yaouba, A.; Serferbe, S. Isolation and identification of fungi associated with avocado fruits from local markets of the West Region of Cameroon. International Journal of Agriculture and Biology, 2015, 4(2), 64-68.
Djeugap, J.F.; Nzong, C.A.; Kyalo, M.; Njukeng, A.P.; Yamdeu, J.H.; Kuiate, J.R.; Ghimire, S. Morphological and molecular identification of pathogenic fungi of Monodora myristica Dunal kernels and their response to different
phytoextracts. International Journal of Advanced Agriculture Research, 2017, 5, 66-75. Djeugap, F.J. Germination constraints and molecular diagnosis of disease-associated fungi in Ricinodendron heudelotii in Cameroon. PhD thesis, University of Laval, Quebec, Canada, 2013, 188p.
Djeugap, F.J. ; Bernier, L. ; Dostaler, D. ; Fontem, D.A. ; Avana, M.L. Germination Constraints on Ricinodendron heudelotii in Cameroon. Seed Technology, 2014, 36 (1), 25-36.
Dongmo, G.Z.; Djeugap, J.F.; Fenohi, N.; Kenfack, N.D.; Takuete, R.; Teguefouet, P. Contribution to the identification of post-harvest fungi associated with Ricinodendron heudelotii and Garcinia kola kernels collected in the West Cameroon Highlands. International Journal of Agriculture and Biosciences, 2017, 11(4), 1840-1850.
Erute, M.O.; Oyibo, A.E. Effects of three plant extracts (Ocimum gratissimum, Acalypha wilkesiana and Acalypha macrostachya) on post-harvest pathogen of Persea americana. Journal of Medical Plants Research, 2008, 2(11), 311-314.
Eyog, M.O.; Ndoye, O.; Kengue, J.; Awono, A. The edible forest fruits of Cameroon, 2006, IPGRI/CIRAD/220 p.
Falleh, H.; Ksouri, R.; Chaieb, K.; Karray-Bouraoui, N.; Trabelsi, N.; Boulaaba, M.; Abdelly, C. Phenolic composition of Cynara cardunculus L. organs, and their biological activities. Biological Reports, 2008, 33, 372–379.
Galani, Y.J.H.; Nguefack, J.; Dakole, D.C.; Fotio, D.; Petchayo, T.S.; Fouelefack, F.R.; Amvam, Z.P.H. Antifungal potential and phytochemical analysis of extracts from seven Cameroonian plants against late blight pathogen Phytophthora infestans. International Journal of Current Microbiology and Applied Sciences, 2013, 2(5), 140-154.
Masete, G.A. In vitro anti-microbial activity of aqueous and ethanolic leaf extracts of Justicia flava and Tephrosia vogelii that grows in Uganda. Student Journal of Health Research, 2021, 13(1): 1-14.
Helal, G.A.; Sarhan, M.M.; Abu Shahla, A.N.K.; Abou El-Khair, E.K. Effects of Cymbopogon citratus L. essential oil on the growth, morphogenesis and aflatoxin production. Journal of Basic Microbiology, 2007, 6(9), 125-131.
Iqbal, N.; Saeed, S. Isolation of mango quick decline fungi from mango bark beetle, Hypocryphalus mangiferae (Coleoptera: Scolytidae). The Journal of Animal and Plant Science, 2012, 22(3), 644-648.
Imathiu, S.M.; Edwards, S.G.; Ray, R.V.; Back, M. Artificial inoculum and inoculation techniques commonly used in the investigation of Fusarium head blight in cereals. Acta Phytopathologica et Entomologica Hungarica, 2014, 49(2), 129-139.
Lulia, G.D.; Matache, M.L.; Tudorache, A.; Chisamer, G.; Rozylowicz, L.; Radu, G.L. Food chain bio magnification of heavy metals in samples from the lower Prut floodplain natural Park. Environmental Engineering and Management Journal, 2012, 11(1), 69-73.
Kausik, M.; Sayan, J.A review on the effects of heavy metals on the aquatic animal of three different districts of West Bengal. Journal of Global Biosciences, 2015, 4(6), 2504-2512.
Kpatinvoh, B.; Adjou, E.S.; Dahouenon-Ahoussi, E.; Konfo, C.T.R.; Atrevi, B.; Soumanou, M. M.; Sohounhloue, D.C.K. Efficacy of essential oils of three aromatic plants against cowpea spoilage mycoflora (Vigna unguiculata L., Walp) collected from retail outlets in southern Benin, Journal of Applied Biosciences, 2007, 109, 10680-10687.
Li, X.; Baskin, J.M.; Baskin, C.C. Seed morphology and physical dormancy of several North American Rhus species (Anacardiaceae). Seed Science Research, 1999, 9, 247-258.
Manfo, D. Practices and challenges of agroforestry in the forest-savannah contact zone: the case of Obala in the Center region of Cameroon. Scientific and Technical Review Congo Basin Forest and Environment, 2018, 11, 66-78.
Mathur, S.B.; Kongsda, O. Methods for detecting Fungi. Common laboratory seed Health Testing/International Seed Testing Association, 2003, 75-77 pp.
Meunier, Q.; Lemmens, R.; Morin, A. Alternatives to exotic species in Uganda-Growth and cultivation of 85 indigenous trees. Kampala, Uganda, French Embassy in Uganda-Belgian Technical Cooperation, 2010, 210p.
Murray, B.I. Plant essential oils for pest and disease management. Crop Protection, 2000, 19, 603-608.
Muyambo, M-A.; Kimoni, K.; Furaha, A.S. Forest products and rural household livelihood strategies in the Kisangani region. Tropenbos DR Congo, 2017, 65p.
Ndoye, O.; Ruiz-Perez, M.; Eyebe, A. The influence of the marketing of non-timber forest products on the degradation of resources in Central Africa: the role of research in the balance between the
well-being populations and the preservation of forests. FAO, 2000, 193 – 217.
Nkongmeneck, B.A.; Nwaga, D.; Ndemmeze, A.; Hallé,
F. Characteristics and germination capacity of seeds of Tetrapleura tetraptera (Schum. & Thonn.) Taub. (Mimosaceae) according to their location in the pod. Revue Ecologique (Terre Vie), 1996, 51(3), 117-124.
Okon, A.E.; Okoi, A.I.; Aboli, O.N.; Obi-Abang, M.; Omini, E.M. Evaluation of two plant extracts for the control of postharvest fungal diseases of cassava (Manihot esculenta Crantz) in Calabar, Nigeria. International Journal of Agricultural Sciences, 2015, 5(3), 487-491.
Onuorah, S.; Orji, M.U. Fungi associated with the spoilage of post-harvest tomato fruits sold in major markets in Awka, Nigeria. International Journal of Microbiology Research, 2015, 3(2), 11-16.
Organization of African Unity. Scientific, Technical, and Research Commission (OAU/STRC). Traditional Medicine and Pharmacopoeia: Contribution to Ethnolobotanical and Floristic Studies in Cameroon edited by Adjanohoun, J.E. Aboubakar, N. Dramane, K., Porto-Novo: OAU/STRC. Available at: https://library.au.int/traditional-medicine-and-pharmacopoeia-contribution-ethnolobotanical-and-floristic-studies-cameroo-5, 1996, pp 512-524.
Pérez-Garcia, F.; Gonzalez-Benito, M.E. Seed germination of five Helianthemum species: Effect of temperature and pre-sowing treatments. Journal of Arid Environment, 2006, 65, 688-693.
Rao, N.K.; Hanson, J.; Dalloo, M.E.; Ghosh, K.; Nowell, D.; Larinde, M. Manual of seed handling in genbanks. Handbooks for Genbanks No. 8 Biodiversity International, Rome, Italy, 2006, 147p.
Salem, M.Z.M.; Mervat, E.H.; Nasser, R.A.; Hayssam, M.A.; Nader, A.E.S.; Hosam, O.E. Medicinal and biological values of Callistemon viminalis extracts: History, current situation and prospects. Asian Pacific Journal of Tropical Medicine, 2017, 27(1), 1-9.
Simons, A.J.; Leakey, R.R.B. Tree domestication in tropical agroforestry. Agroforestry systems, 2004, 61, 167-181.
Sunderland, T.C.H.; Obama, C. Preliminary market study on non-timber forest products in Equatorial Guinea. FAO, 2000, 223-233 pp.
Tabuna, H. The European market for non-timber forest products from Central Africa. FAO, 2000, 267-280 pp.
Wakawa, L.D.; Akinyele, A.O. Effects of pre-treatment on the germination response of old seed of Tetrapleura tetraptera (Schum. and Thonn.) Taub. Journal of Forest Science and Environment, 2016, 1(2), 81 – 86.
Walter, S. Non-wood forest products in Africa: A regional and national overview. EC-FAO partnership program FAO working paper. FOPW/01/, 2001, 303p.
Yaouba, A.; Kemmoe, N.R.; Nchoutnji, I.; Godswill,
N.N. Antifungal potential of Eucalyptus saligna and Cupressus lusitanica extracts against fungi responsible for post-harvest green bean spoilage in Dschang, West Cameroon. Cameroon Journal of Biological and Biochemical Sciences. 2019, 27(2), 29-37.
Yaouba, A.; Tchikoua, R.; Tamsa, A.A.; Mpounze, P. Antifungal activities of some plants extract against Cercospora spp causative agent of postharvest fruits rot. International Journal of Multidisciplinary Research and Development, 2017, 4(2), 200-203.
Yoshimura, H.; Sawai, Y.; Tamotsu, S.; Sakai, A.1, 8-cineole inhibits both proliferation and elongation of BY-2 cultured tobacco cells. Journal of Chemical Ecology. 2010, 37(3), 320-328.




