Hematological and Biochemical Profiles of Exotic and Nigerian Locally Adapted Turkeys Reared in a Tropical Environment
UTULU, Godwin Gbayi; ISIDAHOMEN, Clement Ebanehitah; OGUNTADE, David Oluwafemi; IKHIMIOYA, Imounu
Department of Animal Science, AmbroseAlli University, Ekpoma, Edo State, Nigeria
| ARTICLE INFORMATION | ABSTRACT |
| Corresponding author: Oguntade, David Oluwafemi E-mail: oguntadedo@aauekpoma.edu.ng; davylead@gmail.com Tel: +2348154163003 Live DNA: 234.36633* Keywords: Biochemical parameters Haematological parameters Nicholas white turkey Nigerian locally adapted turkeys | Variations in haematological and biochemical parameters of birds can be used to explain differences in the adaptability and response of different breeds to the local environment. The study aimed at determining the haematological and biochemical variation of Nigerian locally adapted (with different plumage colours) and exotic turkeys reared in tropical environments. A total of 200 day-old poults comprising 150 local (50 Black, 50 Lavender and 50 White) and 50 exotic (Nicholas White) turkeys were used in this study. Blood samples were collected from 180(45 from each genotype) turkeys for the estimation of haematological and biochemical parameters. Genotypes were observed to have a significant (p0.05) to the values in local white and exotic turkeys. The mean corpuscular haemoglobin (MCH), mean corpuscular volume (MCV) and lymphocyte (LYMP) were highest (p0.05) to the values in local white and exotic turkeys. The local white turkey had the highest (p |
INTRODUCTION
Turkeys are classed in the family of Phasianidae in the taxonomic order of Galliformes (Crowe et al. 2006). The origin is somewhat controversial, however, it was reported that the archaeological specimen of wild turkey was found in North America that date to the Pleistocene and turkeys were symbolic of many indigenous groups in North America (Thornton and Emery, 2017) while another view affirmed that the turkey raised in central Mexico was ancestral of all the varieties of domestic turkeys we have today (Dominguez-May et al. 2021). Turkey has gained global recognition due to the high demand for its product (meat) (Yakubu et al. 2013).
The local turkeys found in Nigeria are hardy and often show greater resistance to most tropical poultry diseases. However, they are characterized by small body weight and low production (Ngu et al. 2014). On the other hand, the exotic turkeys had been selected over generations for early maturity and high production of meat (Ilori et al. 2011) but they relatively have low immunity compared to the tropically adapted breeds (Oguntade et al.2021). The immune and health status of animals can greatly influence their productivity and these can be assessed through haematological and biochemical analyses (Abdi Hachesoo et al. 2013).
In addition, blood parameters such as packed cell volume (PCV), red blood cell (RBC) and white blood cell (WBC) will give the idea of feed utilization, cellular respiration and immune condition in animals (Kral and Suchy, 2000).
Evaluation of the haemato-biochemical parameters in birds can be used to determine a response to its internal and external environment (Esonu et al. 2001). The health and functional status of vital body organs (such as the pancreas, heart, muscles, liver and kidney) of an animal can be ascertained through the assessment of plasma or sera components (Agina et al. 2015).
Hence, haematological and biochemical analyses could be used as diagnostic tools to monitor the effectiveness of recommended and applied treatment including determining the toxicity of drugs and chemical substances and forming a prognosis (Alimi et al. 2020) that will curb the future occurrence of ill health. Although, the haematological and serum biochemical parameters of animals are influenced by prevailing environmental and physiological conditions of animals (NseAbasi et al. 2014).
Nevertheless, great variability can be adduced to genotypic differences. Hence, this study would add to the previous knowledge (Ilori et al. 2011; Isidahomen et al. 2013; Ajaonuma et al. 2013; Oguntade et al. 2021) on genetic variation in haematology and biochemistry of tropically adapted and exotic turkeys, which will provide information for the development of tropically adapted broiler turkeys. Therefore, this study assessed the hematological and biochemical profiles of exotic and Nigerian locally adapted turkeys reared in a tropical environment.
MATERIALS AND METHODS
Description of Experimental Site
This research was done at the Turkey Breeding unit of the Teaching and Research Farm of Ambrose Alli University, Ekpoma Edo State, Nigeria. The study which lasted for twenty weeks was carried out between January and June, 2020.
Experimental Birds and Management
A total of two hundred (200) turkeys made up of one hundred and fifty (150) Nigerian locally adapted (50 each of black, lavender and white genotypes) and fifty (50) Nicholas white (exotic) day-old poults were used for this study. The two breeds were sourced from a reputable hatchery in Ibadan, Oyo state Nigeria. The birds were raised in deep litter pens.
They were brooded for a period of four weeks with strict adherence to brooding management practices. The poults were vaccinated against Marek’s disease, Newcastle disease and infectious bronchitis at day old from the hatchery. Subsequent vaccinations and medication were provided routinely and as required. The birds were fed commercially available diets ad libitum and provided constant access to clean water.
All the experimental birds were identified individually through a labelled tag attached to their wings. All the experimental turkeys were maintained under the same experimental management conditions.
Data Collection
Blood samples (about 2.5 ml) were collected from the superficial ulnar vein of 180 turkeys (45 from each genotypic group) at the end of the experiment. About 1 ml of the blood collected was transferred into a tube containing EDTA (ethylene diaminetetracetic acid) for haematological analysis and the other part of the blood was transferred into the plain bottle for biochemical analysis. The routine haematological procedures for avian16 were used to determine the packed cell volume (PCV), red blood cell (RBC), white blood cell (WBC) and differential leukocyte counts. An automated cell counter was used to measure the RBC, haemoglobin (Hb) and WBC within 24 hours after the collection of blood16.
The cyanomethemoglobin method was used to measure the Hb concentration while the PCV was measured by the michrohematocrit method in capillary tubes, centrifuged at 12,000g for 5 minutes (Samour et al. 2010). Other haematological indices such as mean corpuscular volume (MCV), mean corpuscular haemoglobin (MCH) and mean corpuscular haemoglobin concentration (MCHC) were calculated (Samour et al. 2010).
Total protein was determined using the direct biuret method, while the bromocresol green method was used for the determination of serum albumin (Fafiolu and Alabi, 2020). Serum globulin was calculated by subtracting albumin from total protein. Creatinine and urea were determined (Fafiolu and Alabi, 2020). The serum cholesterol and alanine amino transferase (AST and ALT) parameters as well, as alkaline phosphate (ALP) were determined as described by Samour et al. (2010)
Statistical analysis
white turkey (53.22 103μl-1) which is not significantly The data collected were analyzed using the General Linear Model (GLM) procedure of SAS (2014) using the model below:
Yijk = μ + Gi + εij
Where:
Yijk = the dependent variable (haematological and biochemical parameters).
μ = Overall population mean
Gi = Effect of the ith turkey genotypes
εij = The residual random error.
The mean separation was done using Tukey’s HSD of the same software
RESULTS
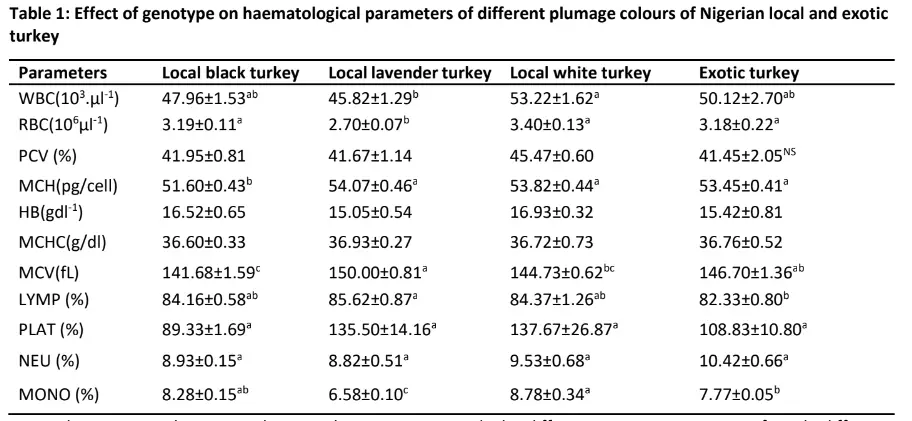
Effect of genotype on hematological parameters of Nigerian local and exotic turkeys Table 1 showed the effect of genotype on turkey haematological parameters of different plumage colours of Nigerian local and exotic turkeys. Genotype had a significant (p
(p>0.05) different from that of local black turkey (47.96103μl-1)and exotic turkey (50.12 103μl-1). The lowest level of WBC was however recorded in local lavender turkey (45.82 103μl-1) which was comparable (p>0.05) with the values recorded in local black and exotic turkey. The highest (p
Conversely, the local lavender had the highest (p0.05) different from the values observed in local black (84.16 %) and white turkey (84.37 %) but significantly (p
Further, the percentage of monocyte was highest (p0.05) different from the level observed in local black turkey (8.28 %) followed by 7.77 % observed in exotic turkey. The local lavender turkey however had the lowest percentage of monocyte (6.58 %).
Note: abc Means in the same column in the same group with the different superscripts are significantly different (P
WBC: White blood cell; RBC: Red blood cell; PCV: Packed cell volume; MCH: Mean cell Haemoglobin; Hb = Haemoglobin Concentration; MCHC: Mean cell Haemoglobin concentration; MCV: Mean cell volume; LYMP: Lymphocyte; PLAT: Platelet; NEU: Neutrophi; MONO: Monocyte.
Effect of genotype on Serum Biochemistry and lipid profile of Nigerian local and exotic turkeys
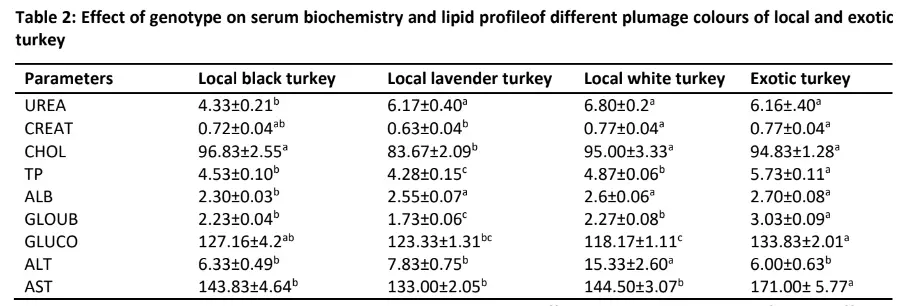
Table 2 showed the effect of genotype on turkey serum biochemistry and lipid profile. Genotype had a significant effect on all the serum biochemistry and lipid profile except the level of alkaline phosphatase (ALP). Genotype significantly (p
Creatinine level was highest (p
The highest level of this lipid was detected in local black (96.83mmol/l) which was not different (p>0.05) from the values in local white (95.00mmol/l)and exotic turkeys (94.83mmol/l) while the lowest level of cholesterol was discovered in local lavender turkey (83.67mmol/l).The total protein value in these turkeys was significantly (p
lavender turkey. Albumin level was also significantly (p0.05) values in local white (2.60 g/l) and lavender (2.55 g/l) turkeys. The level of globulin in the turkeys used in this study followed similar trend as the values obtained in total protein.
In addition, the glucose level was significantly (p0.05) different from the value obtained in local black (127.16 mg/dl) while the least was discovered in local lavender turkey (123.33 mg/dl) which was also similar (p>0.05) to that of local white turkey (118.17 mg/dl).
The highest (p
Note: abc Means in the same column in the same group with the different superscripts are significantly different (P
Urea (mg/dl); CREAT: Creatinine (mg/dl); CHOL: Cholesterol (mmol/l); TP: Total protein (g/l); ALB: Albumin (g/l); GLOUB: Globulin (g/l); GLUCO: Glucose (mg/dl); ALT: Alanine aminotransferase (IU/L); AST: Aspartate aminotransferase (IU/L); ALP: Alkaline phosphatase (IU/L)
DISCUSSION
The result of the haematological profile of local and exotic turkey genotypes in the current study showed that the haematological parameters of turkeys were influenced by the genotypes and feather colour of Nigerian indigenous turkeys. The highest level of WBC
was recorded in local white turkey which is not significantly different from that of local black turkey and exotic turkey but lowest in lavender turkey. This could suggest the better genetic potential of local white turkeys to resist infectious diseases in the tropical climate of Nigeria as they have been naturally selected for adaptation in this environment rather than production.
The major functions of the WBC and its differentials are to fight infections and protect the body against foreign organisms by generating antibodies in immune response (NseAbasi et al. 2014). In addition, animals with low WBCs are exposed to a high risk of disease infection, while those with high WBCs are capable of producing antibodies to resist invasion by pathogens and thereby adapt better to local and disease prevalent environments (NseAbasi et al. 2014). Thus, the local white turkey is superior in terms of survival to the disease prevalent environment.
Generally, the values of WBC obtained for all the turkey genotypes in this study, are in the range of the values reported by Isidahomen et al. (2013) but lower than the values reported by Odunitan-Wayas et al. (2017) for male and female turkeys. The highest level of RBC was recorded in local black and white including exotic turkey while the least was however recorded in the local lavender turkey.
The values of RBC obtained for all the turkey genotypes in the current study fell within the physiological range (2.28—2.81) reported by Daniel-Igwe and Okwara (2018) on turkey. Since RBC helps mainly to take oxygen (through haemoglobin) from the lungs to tissues and removes carbon dioxide out of the body tissues through the lungs (NseAbasi et al. 2014). Therefore, the RBC values in this study implies better chances of healthy living and interaction with the environment in all the turkey genotypes and also signifies that the exotic turkey used in the study are locally adapted to the environment.
Moreover, there observed no significant difference in the level of PCV in the study populations. However, the value of PCV obtained for both local and exotic turkeys were within the range of values (39.77±0.46) for matured turkey as reported by Priya and Gomathy (2008) and (30.66±0.91) reported by Pandian et al. (2012) on Indian turkey. Furthermore, since the PCV is associated with nutritional status (Isidahomen et al. 2013), it is therefore suggested that the values of PCV obtained in the current study for all the turkey genotypes indicate that they were healthy; showed better feed utilization and hence better adaptation to the rearing environment.
In addition, the Hb and MCHC were not significantly influenced by genotype and their values are within the normal range reported by several authors (Bounous and Stedman, 2000). The level of MCH, MCV, LYMP and Mono were significantly influenced by genotype and plumage colour.
The MCV shows the average erythrocyte size while the MCH indicates the haemoglobin amount per blood cell (Bounous and Stedman, 2000). The higher level of the two indices in both local and exotic turkeys except in some feather colour in the Nigerian local turkey suggested that there is no reduction in cellular oxygen requirements in order to adjust to environmental stressors such as anemic disease condition (NseAbasi et al. 2014).
Further, the percentage LYMP was influenced by genotypes with the highest values observed in local turkeys. LYMP changes in concentration in an individual may suggest an interaction between the pathogen and the host. The B lymphocytes are responsible for the rapid generation of antibodies specific to a particular pathogen while T lymphocytes regulate lymphocyte functions and acute viral infection (Pei et al. 2003).In addition, natural variability (Fair et al. 2008) and immune-competence (Berndt and Methner, 2001;Juul-Madsen et al. 2006) were reported in studies of subpopulations of lymphocytes in avian blood.
The values of LYMP in this study reveals immune variability among the four turkey populations while the high level of LYMP in local turkey especially in lavender local turkey suggests that local turkey are much more immune-competent in response to viral infection in the tropical climate when compared to their exotic counterpart. Moreover, monocytes (MONO) are the biggest type of white blood cell and function in the fight against bacteria, viruses and fungi in the body. Their function primarily is to ward off diseases and infections.
These monocytes migrate from the peripheral blood to the tissues and differentiate into macrophages whose morphology and function are dependent on the organ and tissue in which they are present (Geissmann et al. 2010). The level of MONO reported in our work is higher than the level reported by Schmidt et al. (2009) in Bronze turkey which suggests that the turkey used in this study is well adapted to the tropical environment and is able to mount enough immune response against infections that could be brought about by any class of pathogen.
The variation in the levels of urea in the blood of the turkeys with the least value in the black local turkey suggests differences in renal functions associated with metabolic activities in different genotypes of the turkey breeds. Interestingly, creatinine was high in exotic and local white turkey which was not significantly different from those of local black turkey but low in lavender local turkey. Creatinine is the final metabolite of creatine conversion and a major marker of kidney function.
Our results may indicate the better conversion of phosphocreatine to creatinine in the muscle, which suggest reduction in the use of phosphocreatine for muscle contraction. It should be noted that normal functioning of the kidney will result in rapid excretion of Creatinine (Mathuria and Verma, 2008). Increased urea and creatinine as indices of impaired kidney function in aflatoxicosis were reported in chickens and rats (Hassan et al. 2012). However, the levels observed in this study are within the range reported in poultry (Naseem et al. 2018).
Cholesterol was significantly influenced by the genotype of the turkey. The higher level of cholesterol was observed in exotic, local black and local white turkeys while the lavender had the least level of cholesterol. The highly similar pattern of the measured serum cholesterol may be attributed to the possibility of increased metabolism which often influences cholesterol concentration in avian blood serum.
The significantly high level of total protein in the exotic turkey compared to the different indigenous turkeys of different feather colours may be due to improved growth rate coupled with more muscle to the bone ratio in the turkey compared to the indigenous turkey, which is having better ability to convert the feed to muscle than the indigenous turkey. Sera total proteins are currently being used as a diagnostic parameter to ascertain the health condition in birds and together with albumin can give the protein synthesis (Piotrowska et al. 2011). Albumin is high in the turkeys used except in local black turkeys with a lower level.
The values of albumin concentration obtained in this study followed the TP concentration. This suggests the ability of both turkey genotypes to utilize the total available protein from the diets. The level of serum globulin observed with the exotic turkey having the highest level followed by the local black, local white with the least observed in the local lavender turkey may be indicative of an enhanced immune system especially in the exotic turkey as the concentration of serum albumin proteins antioxidant status are regarded as the direct reference to the body immune function (Zhang et al. 2013).
Glucose forms the major source of energy for cellular metabolism. In the current study, the exotic and local black turkeys had higher blood glucose. This slight increase indirectly suggests that these groups possess better absorption of nutrients and enhanced liver glycogenesis (Kokore et al. 2021).
In this study, the serum AST, ALT and ALP differ significantly with turkey genotypes. Higher level of ALP was detected in exotic and local black turkeys. An increase in serum ALP has been attributed to rapid growth and bone activity as reported by Lowe et al. (2022). This is not surprising as the exotic turkey has the highest rate of growth because of its selection for optimum growth when compared with the local counterpart (Ilori et al. 2011).The mean values of ALT and AST are in accordance with the other researchers (Bounous et al. 2000; Ibrahim et al. 2012).
Generally, analysis of blood parameters would provide a reliable indicator of health status of an animal. The values of some blood biochemical parameters namely the glucose, total protein, aspartate aminotransferase (AST), alanine aminotransferase (ALT) and urea; could form a diagnostic tool to confirm various disease conditions and unravel the abnormal function of vital body organs such as liver disorder, kidney disease, diarrhoea and dehydration (Akporhuarho, 2011).
Therefore, information on the blood profiles of various breeds of turkeys could form useful diagnostic tools which would further enhance the development of improved tropical turkey breeds. Although several authors have reported values for the various haematological and serum biochemical parameters of turkey (Patra et al. 2008; Ibrahim et al. 2012), however, there is a need for adequate information on the variability of these values in the different plumage colours as we have in the tropical region such as Nigeria.
CONCLUSION
The current study established genetic variability in haematological and biochemical parameters of Nigerian locally adapted and exotic turkeys. The Nigerian locally adapted turkeys also displayed variations along the three plumage colours. Generally, through haematological and biochemical analysis, it can be deduced from the current study that, the local turkey has a better chance to adapt and survive while the exotic turkey as well is locally adapted to the harsh and disease-prevalent environment of the tropical climate in the study area.
Significance statement
The study discovered that the turkey genotype had a significant effect on all the haematological parameters except Packed Cell Volume (PCV), Hemoglobin (Hb), Mean cell Haemoglobin concentration (MCHC), Platelet (PLAT) and Neutrophil (NEU).
Also, the serum biochemical and lipid parameters examined were significantly affected by genotype, except for the level of alkaline phosphatase (ALP).
Most of the information available on turkey haematology and serum biochemistry was not considered a variation of the values based on plumage colour. Therefore, the variability in haematological and serum biochemical parameters observed among different plumage colours of Nigerian locally adapted turkeys and exotic turkeys would form the basis for screening of Nigerian locally adapted turkeys into different immune-competent groups based on the values of their haematological and biochemical parameters and thus help in the development of tropical broiler-type turkey.
ETHICAL APPROVAL
Ethical clearance was given by the Federal University of Agriculture Abeokuta Ogun State (FUNAAB), Nigeria ethical review board in accordance with international standards on the care and use of experimental animals.
Conflict of interest
The authors declared no conflict of interest.
REFERENCES
Abdi-Hachesoo, B.; Talebi, A.; Asri-Rezaei. S.; Basaki, M,: Sex related differences in biochemical and hematological parameters of adult indigenous chickens in NW of Iran. J. Animal Sci. Ad., 2013, 3, 512-516.
Agina, O.A.; Ezema, W.S.; Nwishienyi, C. Haemato Biochemical Profile of Apparently Healthy Domestic Turkey (Meleagrisgallopavo) in Nsukka, Enugun State. Nigeria Animal Research International, 2015, 12(1), 2120-2129.
Ajaonuma, C.O.; Egahi, J.O.; Zekeri, O.; Ukwenya, S.: The influence of palm kernel cake on haematology and blood chemistry of mixed domesticated turkeys (Meleagrisgallopavo). J. Agric. Vet. Sci., 2013, 2, 1- 3.
Akporhuarho, P.O. Effect of crude oil polluted water on the haematology of cockerel reared under intensive system. International Journal of Poultry Science, 2011, 10(4), 287-289.
Alimi, O.A.; Abdulwahab, W.F.; Amid, S.A.; Abdulkadir, S.Z.; Lawal, F.M.; Aliyu, A.; Adediran, S.O.; Ajadi, A.A.; Bolaji, M.; Uthman, H.O.; Adeyanju, J.B. Hematological prediction study of peritonitis following laparotomy in goats. The Journal of veterinary medical science, 2020, 82(5), 531–535. DOI: 10.1292/JVMS.19-0552.
Berndt, A.; Methner, U. Gamma/delta T cell response of chickens after oral administration of attenuated and non-attenuated Salmonella typhimurium strains. Veterinary Immunology and Immunopathology, 2001, 78 (2), 143-161.
Bounous D.; Stedman, N. Normal avian hematology: chicken and turkey. In: Feldman BF, Zinkl JG, Jain NC, editors. Schalm’s veterinary hematology. New York: Wiley. 2000, 1147-1154.
Bounous, D.I.; Wyatt, R.D.; Gibbs, P.S.; Kilburn, J.V.; Quist, C.F. Normal hematologic and serum biochemical reference intervals for juvenile wild Turkeys. Journal of Wildlife Diseases, 2000, 36, 393-396.
Crowe, T.M.; Bowie, R.C.K.; Bloomer, P.; Mandiwana, T.G.; Hedderson, T.A.J. Phylogenetics, biogeography and classification of/and character evolution in game birds (Aves: Galliformes): effects of character exclusion, data partitioning and missing data. Cladistics, 2006, 33: 495-532.
Daniel-Igwe, G.; Okwara, N. Breed-specific biochemical parameters of healthy adult turkeys in humid tropics in Nigeria. Journal of the Hellenic Veterinary Medical Society, 2018, 68(4), 573-578. DOI: https://doi.org/10.12681/jhvms.16054
Dominguez-May, A.V.; Gamba-Galeazzi, A.P.; Burgos Jimenez, M.N.; Ramirez-Benitez, J.E.; Briceno Narvaez, L.C.; Carrillo-Landell, F.G. The Domestic Turkey (Meleagrisgallopavo) In México. Advances in Agriculture, Horticulture and Entomology 2021(01), AAHE-142. DOI: 10.37722/AAHAE.202111.
Esonu, B.O.; Emenelom, O.O.; Udedebie, U.; Herbert, D.F.; Ekpori, I.C.; Iheukwuemere, F.C. Performance and blood chemistry of weaner pigs fed raw mucuna bean (velvet bean) meal. Tropical Animal Production Investigation, 2001, 4, 49-54.
Fafiolu, A.O.; Alabi, J.O. Diet matrix of stored proprietary feeds: Implications on growth response, health status and carcass yield of broiler chickens. Nigerian Journal of Animal Production, 2020, 47(4), 139-157. DOI. 10.51791/njap.v47i4.82.
Fair, J.M.; Taylor-McCabe, K.J.; Shou, Y.; Marrone, B.L. Immunophenotyping of chicken peripheral blood lymphocyte subpopulations: individual variability and repeatability. Veterinary Immunology and Immunopathology, 2008, 125, 268-273.
Geissmann, F.; Manz, M.G.; Jung, S.; Sieweke, M.H.; Merad, M.; Ley, K. Development of monocytes, macrophages, and dendritic cells. Science (New York, N.Y.), 2010, 327(5966), 656-661. doi:10.1126/science.1178331
Hassan, Z.U.; Khan, M.Z.; Khan, A.; Javed, I.; Hussain, Z. Effects of individual and combined administration of ochratoxin A and aflatoxin B1 in tissues and eggs of White Leghorn breeder hens. Journal of the Science Food Agriculture, 2012, 92, 1540-1544.
Ibrahim A.A, Aliyu J, Abdu M.I, Hassan A.M. Effects of age and sex on serum biochemistry values of Turkeys (Meleagris gallopavo) reared in the semi-arid environment of Nigeria. World Applied Science Journal, 2012, 6, 433-436.
Ilori, B.M.; Peters, S.O.; Yakubu, A.; Imumorin, I.G.; Adeleke, M.A.; Ozoje, M.O.; Ikeobi, C.O.N.; Adebambo, O.A. Physiological adaptation of local, exotic and crossbred turkeys to the hot and humid tropical environment of Nigeria, Acta Agriculturae Scandinavica, Section A – Animal Science, 2011, 61(4), 204-209.
Isidahomen, C. E.; Njidda, A. A.; Amaza, I. B. Effect of genotype on haematology and serum biochemistry values of turkeys (Meleagrisgallopavo) reared in Southern Nigeria. International Journal of Agricultural Biosciences, 2013, 2, 297-301.
Juul-Madsen, H.R.; Dalgaard, T.S.; Rontved, C.M.; Jensen, K.H.; Bumstead, N. Immune response to a killed infectious bursal disease virus vaccine in inbred chicken lines with different major histocompatibility complex haplotypes. Poultry Science, 2006, 85 (6), 986-998.
Kokore, B.A.; Bleyere, N.M.; Kamagate, S.;. YAPO, P.A. Blood Biochemical Parameters Exploration in Broilers and Local Chickens in Korhogo, Côte d’Ivoire. American Journal of Food and Nutrition, 2021, 9(2), 82-86. DOI: 10.12691/ajfn-9-2-4
Kral, I.; Suchy, P. Haematological studies on adolescent breeding cocks. Acta Vet. Brno, 2000, 69, 189-192.
Lowe, D.; Sanvictores, T.; John, S. Alkaline Phosphatase. [Updated 2021 Aug 11]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK459201/
Mathuria, M.; Verma, R. J. Ameliorative effect of curcumin on aflatoxininduced toxicity in serum of mice. Acta Poloniae Pharmaceutica, 2008, 65, 339- 343.
Naseem, M.N.; Saleemi, M.K.; Abbas, R.Z.; Khan, A.; Khatoon, A.; Gul, S.T.; Imran, M.; Sindhu, Z.U.D.; Sultan, A. Hematological and serum biochemical effects of aflatoxin B1 intoxication in broilers experimentally infected with fowl adenovirus-4 (FAdV-4). Pakistan Veterinary Journal, 2018, 38(2), 209-213. http://dx.doi.org/10.29261/pakvetj/2018.028
Ngu, G.T.R.; Butswat, I.S.; Mah, G.D.; Ngantu, H.N. Characterization of small-scale backyard turkey (Meleagrisgallopavo) production system in Bauchi State-Nigeria and its role in poverty alleviation. Livestock Research for Rural Development, 2014, 26 (1).
NseAbasi, N.E.; Williams, M.E.; Akpabio, U.; Offiong, E.E.A. Haematological Parameters and Factors Affecting Their Values Agricultural Science. Published by Science and Education Centre of North America, 2014, 2(1), 37-47.
NseAbasi, N.E.; Williams, M.E.; Akpabio, U.; Offiong, E.E.A.. Haematological Parameters and Factors Affecting Their Values Agricultural Science. Published by Science and Education Centre of North America, 2014, 2(1), 37-47.
Odunitan-Wayas F.; Kolanisi U.; Chimonyo, M. Haematological and Serum Biochemical ResponsesofOvambo Chickens Fed Provitamin A Biofortified Maize. Brazilian Journal of Poultry Science, 2017, 20(3).
Oguntade, D.O.; Ilori, B.M.; Durosaro, S.O.; Abiona, J.A.; Isidahomen, C.E.; Ozoje, M.O. Genetic Variations in Haematological Indices of Local and Exotic Turkeys Inoculated with Attenuated Salmonella typhimurium Vaccine. Asian Journal of Biochemistry, Genetics and Molecular Biology, 2021, 8(1), 19-29.
Panadian,C.M.; ThangaPandiyan.; Sundaresan, A.; Omprakash, A.V.. Haematological Profile and Erythrocyte Indices in Different Breeds of Poultry. International Journal of Livestock Research, 2012, 2, 89-92.
Patra B.; Das S.K.; Mishra P.K.; Mishra S.K.; Panda, N. Evaluation of physio-biochemical traits of growing turkeys in hot and humid climate of Orissa. Indian Journal of Animal Science, 2008, 78, 203-206.
Pei, J.; Briles, W.E.; Collisson, E.W. Memory T cells protect chicks from acute infectious bronchitis virus infection. Virology, 2003, 306, 376-384.
Piotrowska, A.; Burlikowska, K.; Szymeczko, R.. Changes in blood chemistry in broiler chickens during the fattening period. Folia Biol Krakow, 2011, 59(3-4), 183-187.
Priya, M.; Gomatty, V.S. Haematological and blood biochemicals in male and female turkeys of different age groups. Tamilnadu Journal of Veterinary and Animal Science, 2008, 4, 60-68.
Samour, J.H.; Naldo, J.L.; Rahman, H.; Sakkir, M. Haematologic and Plasma Biochemical Reference Values in Indian Peafowl (Pavocristatus). Journal of Avian Medicine and Surgery, 2010, 24, 99-106. https://doi.org/10.1647/2008-019.1
SAS, SAS guide: Statistics released version 9.4M2. Statistical analysis system institute inc.; Cary, NC. https://its.uiowa.edu/sites/its.uiowa.edu/files/SAS %209.4%20System%20Requirements%20Windows .pdf, 2014.
Schmidt, E.M.S.; Paulillo, A.; Lapera, I.; Martins, G.; Junio, L.; Testi, A.; Janine, D.; Lux Hoppe, E.; Fagliari, J. Evaluation of Serum Biochemical Parameters in Juvenile Bronze Turkeys (Meleagris gallopavo). International Journal of Poultry Science, 2009, 8, 746-748.
Thornton, E.K.; Emery, K. F. The Uncertain Origins of Mesoamerican Turkey Domestication. Journal of Archaeological Method and Theory, 2017, 24.
Yakubu A.; Abimiku K.; Musa Azara I.S.; Idahor K.O.; Akinsola, O.M. Assessment of Flock Structure, Preference in Selection and Traits of Economic Importance of Domestic Turkey (Meleagris gallopavo) Genetic Resources in Nasarawa State, Nigeria. Livestock Resource and Rural Development, 2013, 25(1), 18.
Zhang, H.; Chen, H.; Zhang, Y.; Li, S.; Lu, D.; Zhang, H.; Meng, Z.; Liu, X.; Lin, H. Molecular cloning, characterization and expression profiles of multiple leptin genes and a leptin receptor gene in orange spotted grouper (Epinephelus coioides). Gen Comp Endocrinol, 2013, 181, 295-305.

